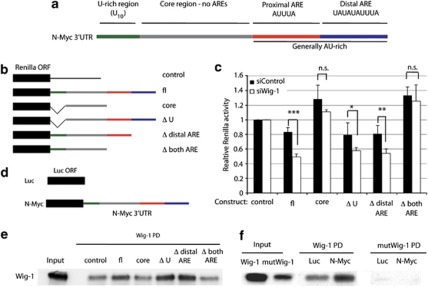
Figure 2.
Wig-1 regulates N-Myc mRNA by binding to the 3′UTR. The N-Myc 3′UTR contains a U-rich stretch and a general AU-rich region containing two canonical AREs (a). To determine if Wig-1 regulates N-Myc through any of these regions, we generated the constructs in (b) linked to renilla luciferase and assessed renilla activity compared with control luciferase activity. (c) Statistically significant reduction after Wig-1 knockdown compared with control siRNA of relative renilla activities was observed with the constructs containing the proximal ARE, that is, full-length N-Myc 3′UTR (fl) (P=7.3 × 10−5); Δ distal ARE (P=0.006); and ΔU (P=0.044), but not with the constructs lacking the proximal ARE (core and Δ both ARE, n.s. stands for not significant) (n=4). To determine if Wig-1 binds to the N-Myc 3′UTR – and in that case, identify the region required for binding – we used the constructs in panel (b) as templates for making biotinylated RNA probes that were mixed with cell lysates from HCT116 cells ectopically expressing wild-type Wig-1. Biotinylated probes were captured using streptavidin beads, washed, and bound proteins were analyzed by western blotting. (e) Wig-1 binds to the probes generated from constructs in panel (b) that contain the proximal ARE but not to those lacking it. PD=pulldown. To investigate if the RNA binding capacity of Wig-1 is required for the binding to N-Myc mRNA, we used luciferase constructs either without a 3′UTR (‘control') or with the 3′UTR of N-Myc (‘N-Myc') (d) as templates for making biotinylated RNA probes that were mixed with cell lysates from HCT116 cells ectopically expressing either wild-type Wig-1 (‘Wig-1') or a zinc finger point mutant form of Wig-1 that cannot bind to RNA10 (‘mutWig-1') and performed the pulldown as above. (f) Binding of wild-type but not mutant Wig-1 to the 3′UTRs of N-Myc, but not to the control RNA. Densitometry analysis using ImageJ showed 1.4 times more wt Wig-1 than mutant Wig-1 protein in the input, most likely reflecting decreased protein stability of the mutant protein. However, 64.5 times more wt than mutant Wig-1 was bound to the N-Myc probe (f)