To the Editor: In a recent issue of Emerging Infectious Diseases, Seuberlich et al. (1) reported a novel prion protein in cattle with bovine spongiform encephalopathy (BSE). Two cows in Switzerland, 8 and 15 years of age, tested positive in 2 approved screening tests, the PrioSTRIP test and the Prionics Check WESTERN (Prionics, Zurich, Switzerland). According to World Organisation for Animal Health guidelines, the 2 cattle are considered BSE positive. Histopathologic and immunohistochemical results were inconclusive because the tissues were severely autolyzed. Clinical signs were absent or the clinical history was not known.
After further analysis of brain tissues by using several monoclonal antibodies in a Western blot (WB), the authors concluded that they had identified an N-terminal truncated protease-resistant prion protein (PrPres) fragment that differs from the PrPres fragments in 3 known types of BSE. No reference was made to the existence of N-truncated fragments, such as C1, of the normal prion protein PrPC, which have been reported for humans (2,3), mice (4), and cattle and other ruminants (5). The pattern in the WB of the novel prion protein (1) appears similar to that of the fragment C1 of the normal prion protein (2–5). The C1 fragment is more protease resistant than the intact PrPC fragment because the protein part is more protected by the polysaccharide residues. Could it be that in the case of the severely autolyzed tissues of the cows in Switzerland, the proteinase K might already have been weakened or inhibited and when combined with the higher protease resistance of the C1 fragment, the digestion was incomplete?
Ten years ago, I looked at nonspecific, unusual samples from fallen stock cattle in New Zealand. Samples from these cattle had been confirmed as negative by paraffin-embedded tissue blot (University of Göttingen, Göttingen, Germany), sodium phosphotungstic acid precipitation, followed by WB (European Union Reference Laboratory for Transmissible Spongiform Encephalopathies, Veterinary Laboratories Agency, New Haw, UK), Prionics WB (Prionics AG, Zurich, Switzerland), histopathologic examination, and immunohistochemical testing (Veterinary Laboratories Agency). We became aware of such samples when the proteinase K digestion did not work properly (Figure). Unusual samples 1 and 2 contained increased amounts of a truncated fragment of normal PrPC, which was digested completely after the proteinase K concentration was increased.
Figure.
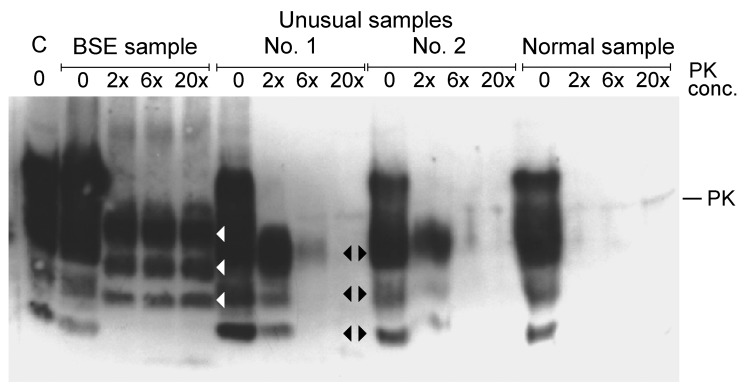
Western blot analysis of proteinase K (PK) digested brain stem samples with increasing concentrations of PK (relative to concentration used in the Prionics Check WESTERN (Prionics, Zurich, Switzerland). C, kit control, normal bovine brain homogenate; BSE, bovine spongiform encephalopathy sample from cow from Switzerland; unusual samples 1 and 2 and normal sample are from New Zealand cattle and had been confirmed as negative by several test methods (see text). Unusual samples 1 and 2 show a higher concentration of a truncated fragment of the normal prion protein, which was completely digested at increased PK concentrations. It is typical for this fragment that bands are identical in size to lower bands of the normal prion protein in the undigested samples of normal brain homogenates (black arrowheads). White arrowheads show major bands of PK-digested BSE prion protein.
I am convinced that the novel PrPres described in article by Seuberlich et al. (1) is indeed a truncated fragment of the normal bovine PrPC protein. Therefore, I would like to ask the editor to address the following issues with the authors: Why were no references to truncated fragments of PrPC made in their article? Why was no WB analysis performed in which the novel PrPres was shown next to normal, undigested PrPC for band-size comparison? Why were no WB analyses shown in which the proteinase K concentration was increased?
It is laudable that in vivo transmission studies using transgenic mouse models and cattle are under way, which will sort out these findings conclusively. I expect that no disease development will be shown. Meanwhile, announcing new types of BSE is purely speculation.
Footnotes
Suggested citation for this article: Kittelberger R. Novel prion protein in BSE-affected cattle, Switzerland [letter]. Emerg Infect Dis [serial on the Internet]. 2012 May [date cited]. http://dx.doi.org/10.3201/eid1805.111824
References
- 1.Seuberlich T, Gsponer M, Drögemüller C, Polak MP, McCutcheon S, Heim D, et al. Novel prion protein in BSE-affected cattle, Switzerland. Emerg Infect Dis. 2012;18:158–9. 10.3201/eid1801.111225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio-Gambetti L. Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem. 1995;270:19173–80. 10.1074/jbc.270.32.19173 [DOI] [PubMed] [Google Scholar]
- 3.Pan T, Li R, Wong B-S, Liu T, Gambetti P, Sy M-S. Heterogeneity of normal prion protein in two-dimensional immunoblot: presence of various glycosylated and truncated forms. J Neurochem. 2002;81:1092–101. 10.1046/j.1471-4159.2002.00909.x [DOI] [PubMed] [Google Scholar]
- 4.Mangé A, Béranger F, Peoc’h K, Onoder T, Frobert Y, Lehmann S. Alpha- and beta- cleavages of the amino-terminus of the cellular prion protein. Biol Cell. 2004;96:125–32. 10.1016/j.biolcel.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Klingeborn M. The prion protein in normal cells and disease. Studies on the cellular processing of bovine PrPC and molecular characterization of the Nor98 prion [dissertation]. Uppsala (Sweden): Swedish University of Agricultural Sciences; 2006.


