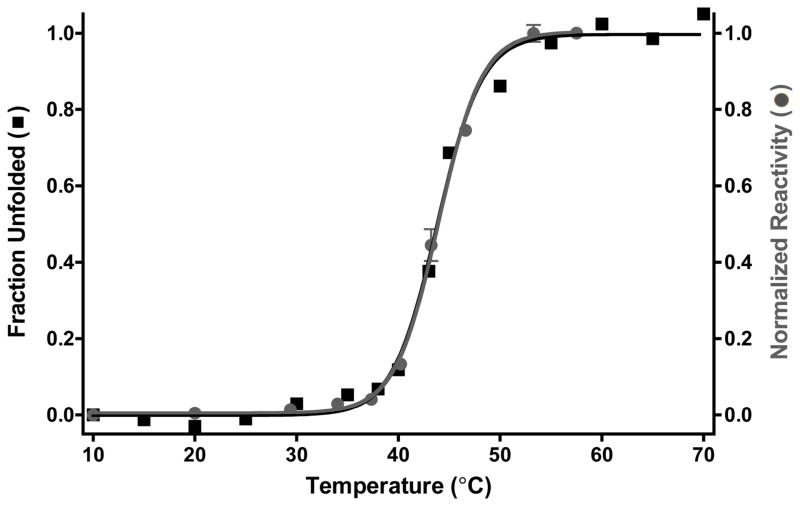
Figure 3.
Ubc4′ becomes a substrate of HsQSOX upon thermal unfolding. The thermal denaturation profile for Ubc4′ was monitored by circular dichroism at 222 nm (squares, see Methods) and the change normalized as shown. Initial rates for Ubc4′ oxidation were established at the indicated temperatures using a discontinuous assay (circles; see Methods). Control experiments omitting QSOX were performed at each temperature and were subtracted from each reaction. Enzymatic activity was normalized to 1.0 at 60 °C as shown.