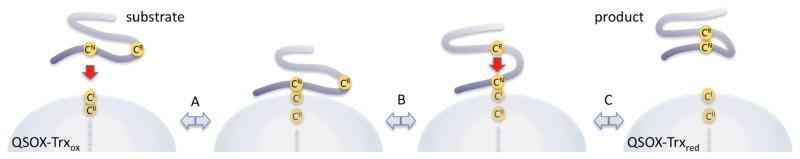
Figure 9.
Oxidation of a flexible protein substrate by the redox-active Trx domain of QSOX. The nucleophilic cysteine sulfur atom in a flexible protein substrate is depicted by CN and that of the corresponding resolving cysteine by CR. Each disulfide exchange step requires the reacting sulfur atoms to achieve approximate colinearity. Following an initial covalent capture of the substrate in step A, a conformational change in the substrate (step B) is required to bring the resolving sulfur atom in line with the CN-CI mixed disulfide.