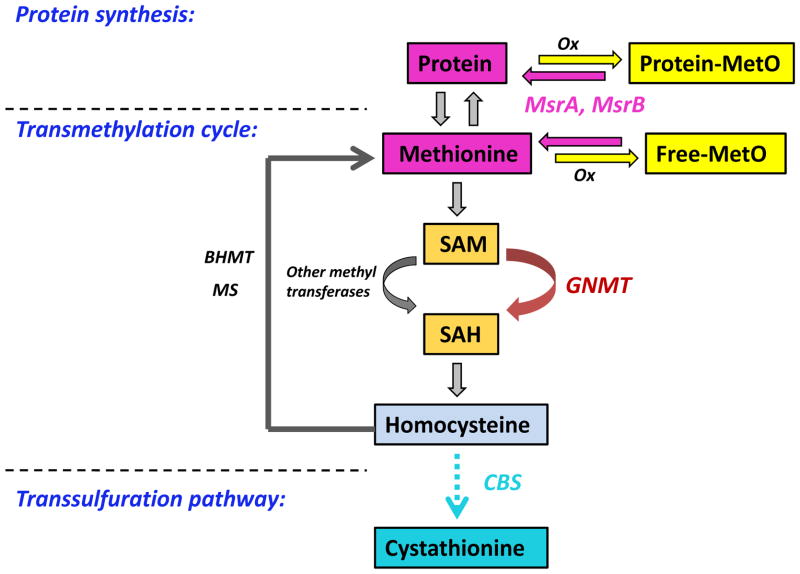
Figure 1.
Key components in the regulation of methionine metabolism. In vivo, both free and protein bound methionine can be oxidized by reactive oxygen and nitrogen species. msrA and msrB can reverse the methionine oxidation to replenish the methionine pool. The vast majority of methionine is found in proteins. The most important role of methionine in intermediary metabolism is in one carbon transfer. Methionine is required for generation of SAM, the major biological methyl donor in vivo. In the transmethylation cycle, SAM is converted to SAH, primarily by GNMT in the liver. SAH is further hydrolyzed to form homocysteine, which can be remethylated to regenerate methionine by MS or BHMT. Homocysteine can also undergo an irreversible transsulfuration pathway to form cystathionine. This also occurs mainly in the liver and is catalyzed by the enzyme CBS. The abbreviations are: MS, methionine synthase; BHMT, betaine-homocysteine methyltransferase; GNMT, glycine N-methyltransferase; CBS, cystathionine –synthase; Ox, oxidizing species; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine