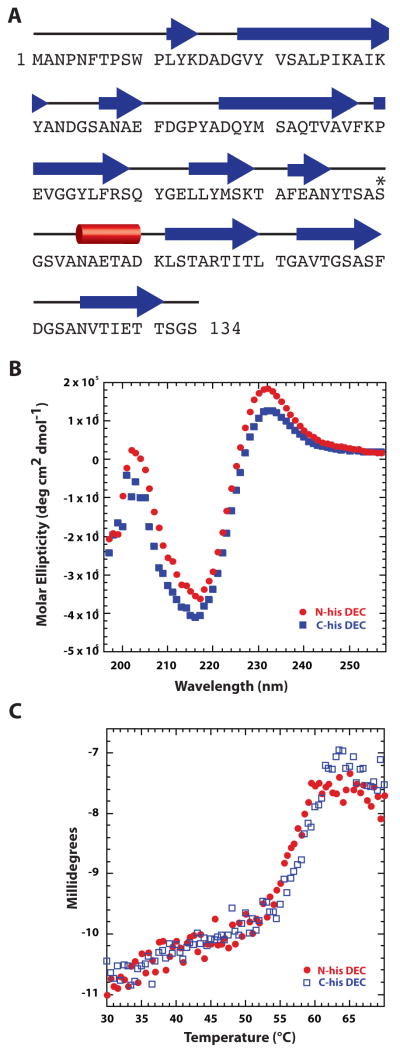
Figure 3. Dec is a β-rich protein.
A. Secondary structure of Dec predicted by PsiPred [62] shows high β content. Other programs like SABLE [63] gave similar results. Individual β-strands are depicted by blue arrows, and the lone helix is represented by a red cylinder. The asterisk identifes S90, which was engineered to cysteine for anisotropy experiments. B. Circular dichroism spectra of the N- and C-terminal, hexa-histidine-tagged Dec protein. C. Circular dichroism spectra at 218 nm monitored during a thermal melting experiment in which the sample was raised from 30 to 70 °C at a rate of 0.3 °/min.