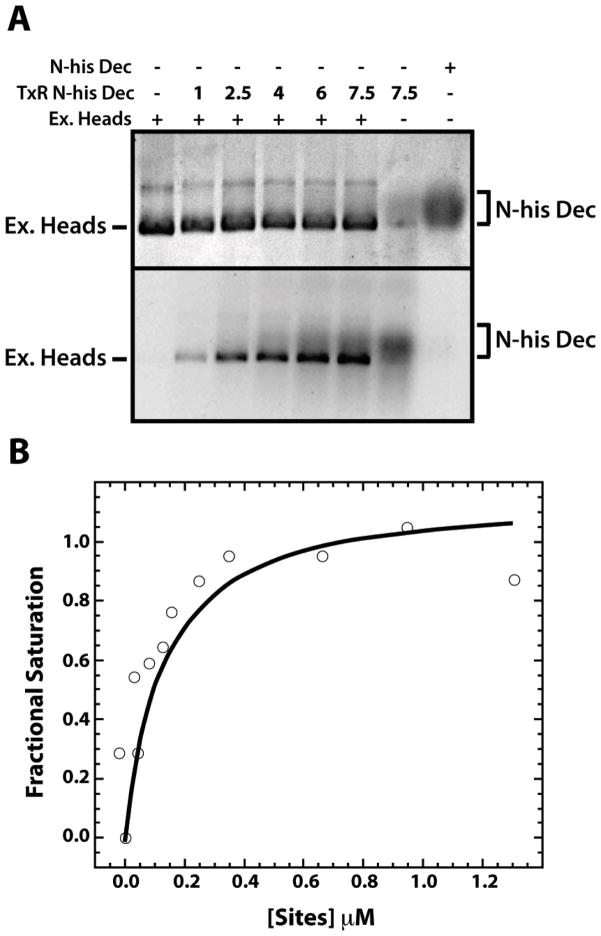
Figure 4. High affinity binding of Dec trimers to ExH.
A. ExH at 0.027 μM (equivalent to 1.6 μM Dec binding locations, considering only the 60 high affinity sites at quasi-three fold symmetry axes) were mixed with Texas Red labeled, N-his-tagged, S90C Dec (TxR N-his Dec) at increasing μM concentrations, as indicated in the second line of the caption to the gel, at increasing concentrations, as indicated, or with unlabeled, N-his-tagged Dec. Samples were run on a native agarose gel and visualized by Coomassie Blue staining (top) or by fluorescence (excited with a transilluminator at 280 nm) (bottom). B. A representative fluorescence anisotropy assay of the binding of Texas Red labeled, N-his S90C Dec (at a concentration of 0.3 μM), was monitored during a titration with ExH. The abcissa axis gives the μM concentration of ExH multiplied by 60.