Abstract
A large proportion of newly synthesized polyribosomal RNA of sea urchin blastulae is not polyadenylylated. The size distributions of the polyadenylylated and nonpolyadenylylated RNA are indistinguishable (mean size of 26 S). Upon translation of sea urchin polyribosomal RNA containing poly(A) and that without poly(A) in a wheat embryo cell-free protein-synthesizing system, where polypeptide synthesis is dependent on added messenger, both classes of RNA support peptide synthesis to the same extent. A preliminary analysis of the proteins synthesized in response to added mRNA (polyadenylylated and nonpolyadenylylated) indicates that these classes of mRNA molecules may code for different populations of proteins.
Full text
PDF
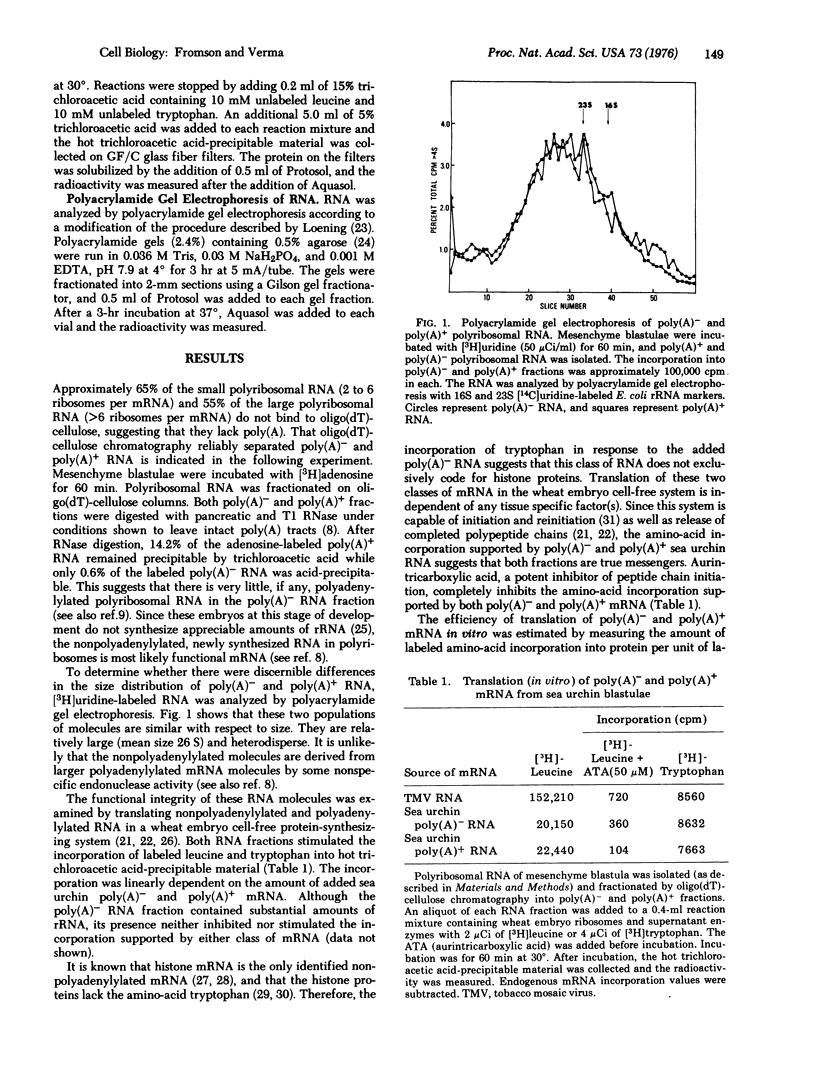
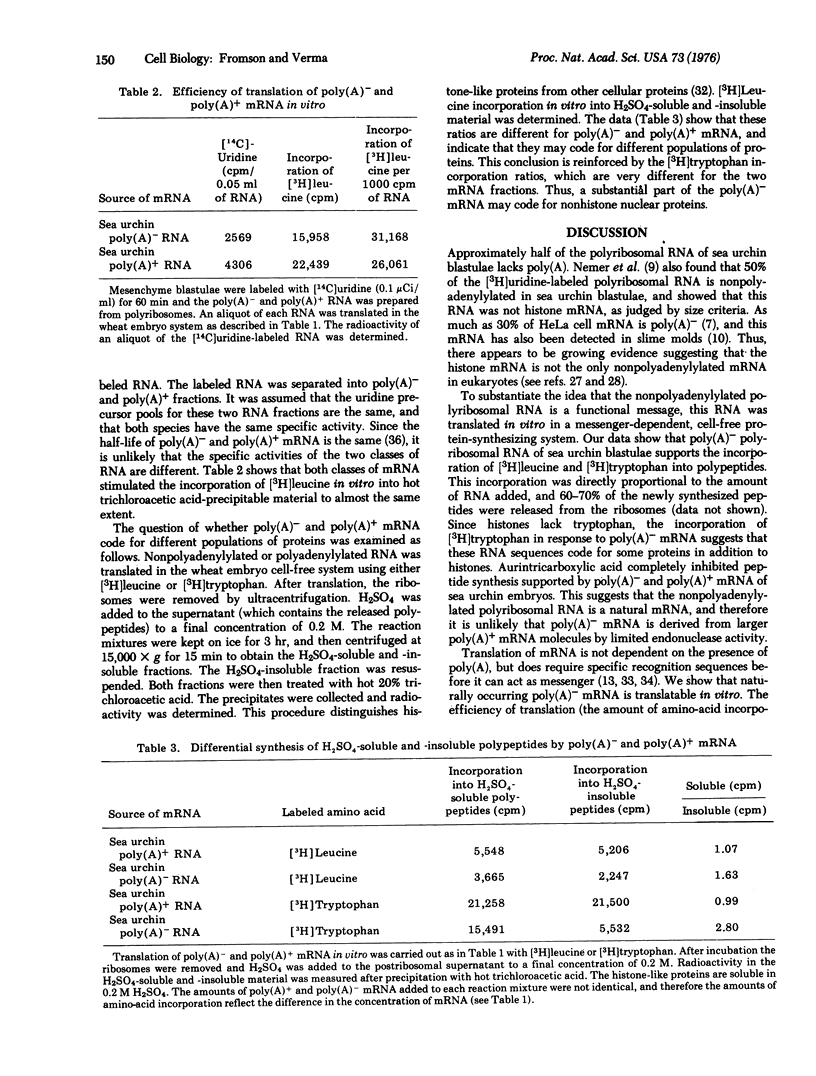

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Burr H., Lingrel J. B. Poly A sequences at the 3' termini of rabbit globin mRNAs. Nat New Biol. 1971 Sep 8;233(36):41–43. doi: 10.1038/newbio233041a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Humphreys T. Regulation of DNA-like RNA and the apparent activation of ribosomal RNA synthesis in sea urchin embryos: quantitative measurements of newly synthesized RNA. Dev Biol. 1970 Sep;23(1):86–112. doi: 10.1016/s0012-1606(70)80008-2. [DOI] [PubMed] [Google Scholar]
- Fernandez Muñoz R., Darnell J. E. Poly(A) in mRNA does not contribute to secondary structure necessary for protein synthesis. Cell. 1974 Aug;2(4):247–252. doi: 10.1016/0092-8674(74)90018-x. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Jacobson A., Lodish H. F. Isolation and hybridization kinetics of messenger RNA from Dictyostelium discoideum. Nat New Biol. 1972 Oct 25;239(95):225–228. doi: 10.1038/newbio239225a0. [DOI] [PubMed] [Google Scholar]
- Fromson D., Duchastel A. Poly (A)-containing polyribosomal RNA in sea urchin embryos: changes in proportion during development. Biochim Biophys Acta. 1975 Feb 10;378(3):394–404. doi: 10.1016/0005-2787(75)90184-7. [DOI] [PubMed] [Google Scholar]
- Fromson D., Nemer M. Cytoplasmic extraction: polyribosomes and heterogenous ribonucleoproteins without associated DNA. Science. 1970 Apr 10;168(3928):266–267. doi: 10.1126/science.168.3928.266. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Messenger RNA metabolism of animal cells. Possible involvement of untranslated sequences and mRNA-associated proteins. J Cell Biol. 1975 Feb;64(2):269–288. doi: 10.1083/jcb.64.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Relative occurrence of polyadenylic acid sequences in messenger and heterogeneous nuclear RNA of L cells as determined by poly (U)-hydroxylapatite chromatography. J Mol Biol. 1972 Dec 14;72(1):91–98. doi: 10.1016/0022-2836(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Leclercq M., Nudel U., Soreq H., Salomon R., Lebleu B., Revel M., Littauer U. Z. Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3143–3146. doi: 10.1073/pnas.71.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Gross P. R. Synthesis and function of messenger RNA during early embryonic development. J Mol Biol. 1969 Jun 28;42(3):559–575. doi: 10.1016/0022-2836(69)90243-5. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Jacobson A., Firtel R., Alton T., Tuchman J. Synthesis of messenger RNA and chromosome structure in the cellular slime mold. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5103–5108. doi: 10.1073/pnas.71.12.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Moav B., Nemer M. Histone synthesis. Assignment to a special class of polyribosomes in sea urchin embryos. Biochemistry. 1971 Mar 2;10(5):881–888. doi: 10.1021/bi00781a024. [DOI] [PubMed] [Google Scholar]
- Murray K. The acid extraction of histones from calf thymus deoxyribonucleoprotein. J Mol Biol. 1966 Feb;15(2):409–419. doi: 10.1016/s0022-2836(66)80116-x. [DOI] [PubMed] [Google Scholar]
- Nemer M., Dubroff L. M., Graham M. Properties of sea urchin embryo messenger RNA containing and lacking poly(A). Cell. 1975 Oct;6(2):171–178. doi: 10.1016/0092-8674(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Graham M., Dubroff L. M. Co-existence of non-histone messenger RNA species lacking and containing polyadenylic acid in sea urchin embryos. J Mol Biol. 1974 Nov 5;89(3):435–454. doi: 10.1016/0022-2836(74)90474-4. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Greenberg J. R., Kelley D. E., LaTorre J., Schochetman G. Messenger RNA: its origin and fate in mammalian cells. Basic Life Sci. 1973;1:149–168. doi: 10.1007/978-1-4684-0877-5_12. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Schodhetman G., Perry R. P. Early appearance of histone messenger RNA in polyribosomes of cultured L cells. J Mol Biol. 1972 Feb 14;63(3):591–596. doi: 10.1016/0022-2836(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Marcus A. Preformed messenger of quiescent wheat embryos. Biochim Biophys Acta. 1971 Apr 8;232(4):671–684. doi: 10.1016/0005-2787(71)90759-3. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Verma D. P., Seal S. N., Marcus A. Role of ribosomal subunits in eukaryotic protein chain initiation. Nature. 1972 Mar 24;236(5343):167–168. doi: 10.1038/236167a0. [DOI] [PubMed] [Google Scholar]
- Williamson R., Crossley J., Humphries S. Translation of mouse globin messenger ribonucleic acid from which the poly(adenylic acid) sequence has been removed. Biochemistry. 1974 Feb 12;13(4):703–707. doi: 10.1021/bi00701a011. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2345–2349. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]


