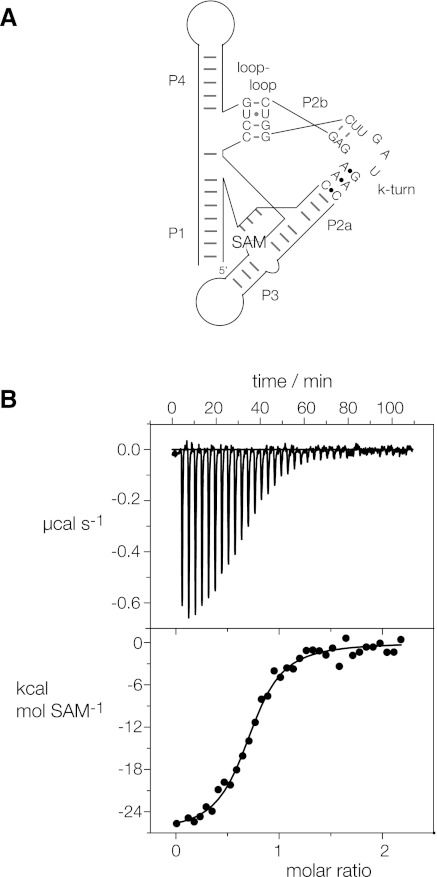
FIGURE 3.
Isothermal titration calorimetric analysis of SAM binding to the SAM-I riboswitch in which the k-turn has been replaced by that of T. solenopsae Kt-23. A solution of SAM was titrated into a SAM-I riboswitch RNA solution, and the heat evolved was measured by ITC as the power required to maintain zero temperature difference with a reference cell. Integration over time gives the heat required to maintain thermal equilibrium between cells. (A) Scheme of SAM-I riboswitch with T. solenopsae Kt-23. (B) Calorimetric data. The upper panel shows the raw data for sequential injections of 8-μL volumes (following an initial injection of 1 μL) of a 100 μM solution of SAM into a 1.4 mL 10 μM RNA solution in 50 mM HEPES (pH 7.5), 100 mM KCl, 10 mM MgCl2. This represents the differential of the total heat (i.e., enthalpy ΔH° under conditions of constant pressure) for each SAM concentration. The lower panel presents the integrated heat data fitted to a single-site binding model (Equation [3]). The thermodynamic parameters calculated are summarized in Table 2.