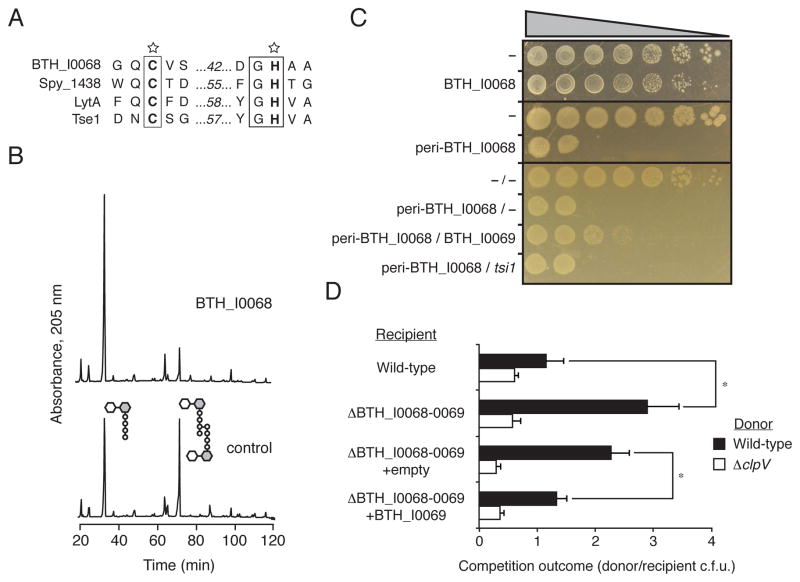
Figure 2. B. thailandensis BTH_I0068 and BTH_I0069 are a T6S amidase effector–immunity pair.
(A) Sequence alignment of conserved catalytic motifs share between BTH_I0068 and characterized cell wall amidase enzymes. SWISS-PROT entry names for the proteins shown are: BTH_I0068 (Q2T2K7_BURTA), Spy_1438 (Q99Z24_STRP1), LytA (LYTA_BACSU), and Tse1 (Q9I2Q1_PSEAE).
(B) BTH_I0068 acts as a peptidoglycan amidase with specificity toward the m-DAP-D-alanine DD-bond. Partial HPLC chromatograms of sodium borohydride-reduced soluble E. coli peptidoglycan products resulting from digestion with BTH_I0068 and subsequent cleavage with cellosyl.
(C) Growth of E. coli harboring one (top panels) or two (bottom panel) vectors expressing the indicated genes. A dash indicates the empty vector. From left to right are increasing serial ten-fold dilutions. Expression data for this experiment are shown in Figure S2.
(D) BTH_I0068 and BTH_I0069 act between cells as a T6S-dependent toxin–immunity pair between B. thailandensis cells. Growth competition assays between the indicated B. thailandensis donor and recipient strains under T6S-conducive conditions. The ΔclpV1 strain is a T6S-deficient control. Asterisks mark significantly different competition outcomes (p < 0.01). Error bars represent ± s.d. n=6.