Fig. 1.
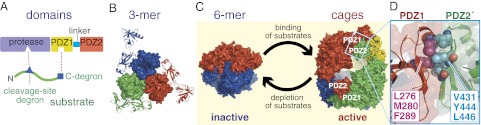
Domain and oligomeric structures of DegP. (A) DegP subunits contain a protease domain and two PDZ domains. Simple model substrates contain a C-terminal degron that binds to PDZ1 and a cleavage-site degron that binds to the active site of the protease domain. (B) A DegP trimer is the fundamental unit of assembly. This trimer is stabilized by packing between protease domains, which are shown in surface representation and colored individually. The PDZ1 and PDZ2 domains are on the periphery of the trimer and are shown in cartoon representation in the same color as the attached protease domain. The trimer shown consists of subunits A, B, and C from PDB structure 3OTP (10). (C) Upon addition of substrate, DegP is transformed from a proteolytically inactive hexamer to larger cages containing 4, 6, 8, or more trimers (8–10). Each trimer in the hexamer (1KY9) and the dodecamer (3OTP) shown is displayed in surface representation and is a different color. After the substrate is degraded, cages dissociate back to inactive hexamers (10). (D) Close-up view of PDZ1–PDZ2′ contact, emphasizing PDZ1 residues (L276, M280, and F289) and PDZ2′ residues (V431, Y444, and L446) that pack together in the hydrophobic interface (shown in Corey–Pauling–Koltun representation).