Abstract
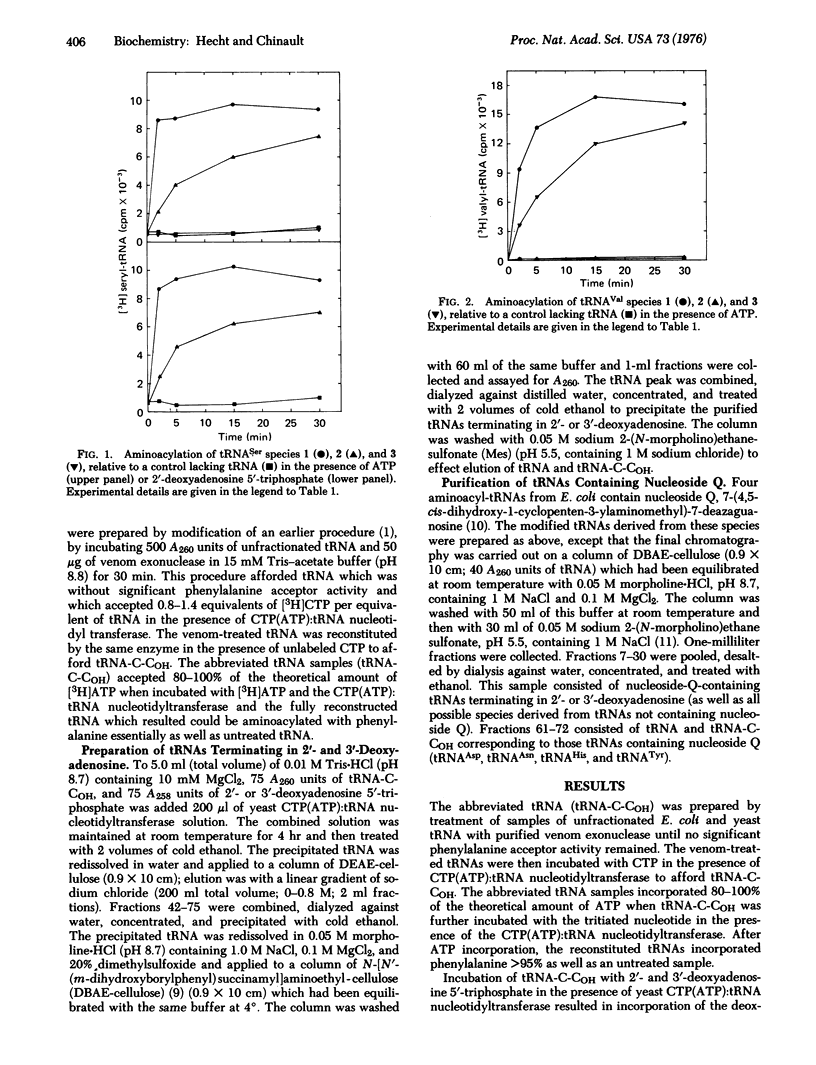
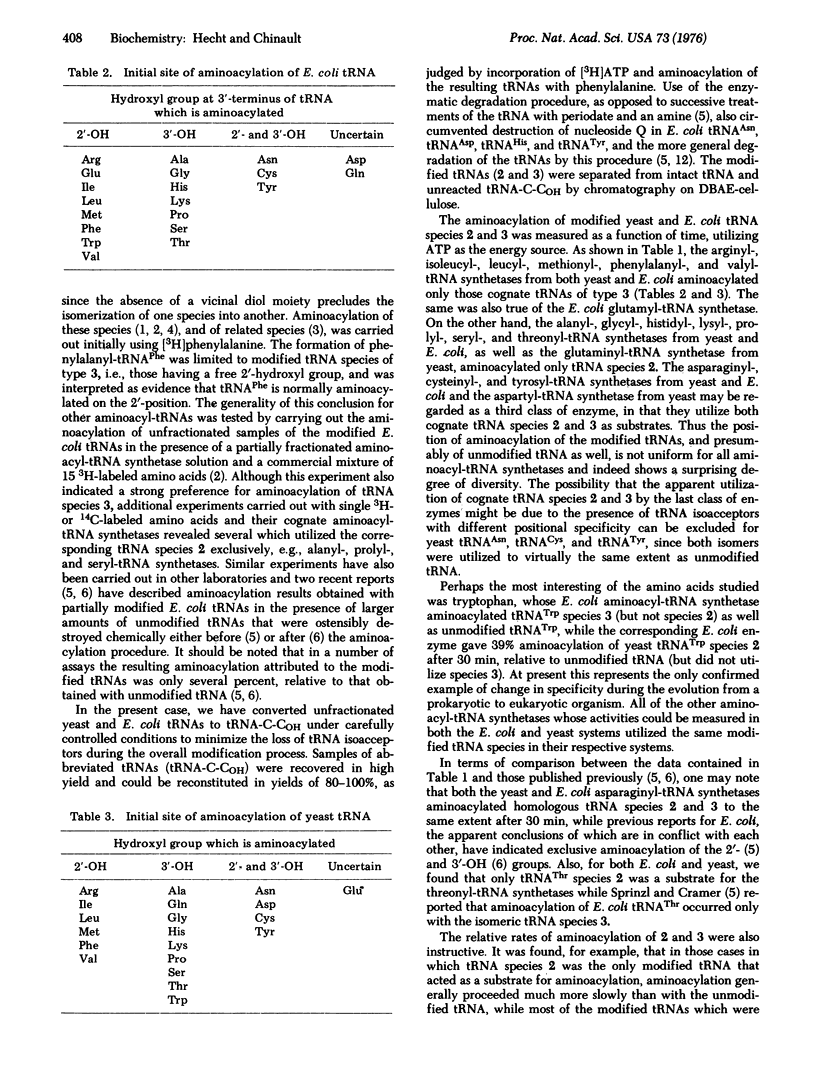
Transfer RNAs terminating 2'-or 3'-deoxyadenosine were prepared from unfractionated E. coli and yeast (Saccharomyces cerevisiae) tRNAs and purified to remove unmodified tRNAs. The modified tRNA species were assayed for aminoacylation with each of the 20 amino acids to determine the initial position of tRNA aminoacylation. The E. coli and yeast aminoacyl-tRNA synthetases specific for arginine, isoleucine, leucine, methionine, phenylalanine, and valine, as well as the E. coli glutamyl-tRNA synthetase, aminoacylated only those cognate tRNAs terminating in 3'-deoxyadenosine (i.e., those having a 2'-OH group). On the other hand, those E. coli and yeast synthetases specific for alanine, glycine, histidine, lysine, proline, serine, and threonine, as well as the yeast synthetase specific for glutamine, utilized exclusively those tRNAs having an available 3'-OH group on the 3'-terminal nucleoside, while the E. coli and yeast synthetases specific for asparagine, cysteine, and tyrosine, and the yeast aspartyl-tRNA synthetase, utilized both of the modified cognate tRNAs. The only observed difference in specificity between the E. coli and yeast systems was for tRNATrp, which was aminoacylated on the 2'-position in E. coli and the 3'-position in yeast. The results indicate that the initial position of aminoacylation is not uniform for all tRNAs, although for individual tRNAs the specificity has been conserved during the evolution from a prokaryotic to eukaryotic organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinali G., Sprinzl M., Parmeggiani A., Cramer F. Participation in protein biosynthesis of transfer ribonucleic acids bearing altered 3'-terminal ribosyl residues. Biochemistry. 1974 Jul 16;13(15):3001–3010. doi: 10.1021/bi00712a001. [DOI] [PubMed] [Google Scholar]
- Fraser T. H., Rich A. Amino acids are not all initially attached to the same position on transfer RNA molecules. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3044–3048. doi: 10.1073/pnas.72.8.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser T. H., Rich A. Synthesis and aminoacylation of 3'-amino-3'-deoxy transfer RNA and its activity in ribosomal protein synthesis. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2671–2675. doi: 10.1073/pnas.70.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. M., Hawrelak S. D. Interaction of glycyl-L-phenylalanine with Escherichia coli phenylalanyl-tRNA synthetase. Biochemistry. 1974 Nov 19;13(24):4967–4975. doi: 10.1021/bi00721a015. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Hawrelak S. D., Kozarich J. W., Schmidt F. J., Bock R. M. Chemical modifications of transfer RNA species. Transfer RNA's terminating in 2'- and 3'-O-methyladenosine. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1341–1347. doi: 10.1016/0006-291x(73)90648-7. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Kozarich J. W., Schmidt F. J. Isomeric phenylalanyl-tRNAs. Position of the aminoacyl moiety during protein biosynthesis. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4317–4321. doi: 10.1073/pnas.71.11.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975 Sep 23;14(19):4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Chinault A. C., Hecht S. M. Ribonucleoside phosphates via phosphorimidazolidate intermediates. Synthesis of pseudoadenosine 5'-triphosphate. Biochemistry. 1973 Oct 23;12(22):4458–4463. doi: 10.1021/bi00746a024. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. Site of aminoacylation of tRNAs from Escherichia coli with respect to the 2'- or 3'-hydroxyl group of the terminal adenosine. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3049–3053. doi: 10.1073/pnas.72.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal J., Deutscher M. P., Littauer U. Z. Biological activity of Escherichia coli tRNA Phe modified in its C-C-A terminus. Eur J Biochem. 1972 Aug 4;28(4):478–491. doi: 10.1111/j.1432-1033.1972.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Weith H. L., Wiebers J. L., Gilham P. T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry. 1970 Oct 27;9(22):4396–4401. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]


