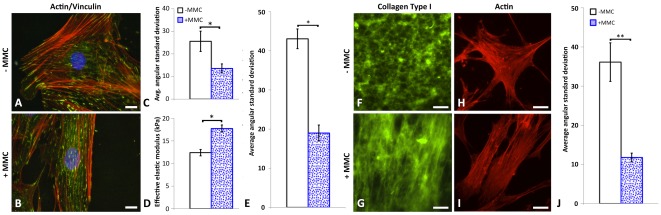
Figure 2. Macromolecular crowding directly alters organization of deposited extracellular matrix proteins and thus alters the orientation of the actin cytoskeleton.
(A) Immunostaining of intracellular F-actin (red), intracellular vinculin (green) as a focal adhesion protein involved in the linking of integrin to actin cytoskeleton, and nucleus (blue, DAPI) of human bone marrow-derived mesenchymal stromal or stem cells (MSCs) after 3 days of cell culture in media containing macromolecular crowders (+MMC media) and (B) −MMC media. Scale bars = 30 µm. (C) Quantification of average angular standard deviation for F-actin (N = 10 +MMC, N = 10 −MMC, p = 0.0223) where lower values indicate a higher degree of alignment. (D) Effective Young's elastic modulus in kPa measured by atomic force microscopy enabled nanoindentation of MSCs ± MMC suggests a stiffening of the cortical cytoskeleton +MMC. (E) Average angular standard deviation of FITC-conjugated rat tail type-I collagen network deposited on plasma treated glass coverslips, (F) in media absent of macromolecular crowders (−MMC) and, (G) +MMC. Scale bars = 25 µm. (H) Immunostaining of F-actin (red) after 3 days for human bone marrow-derived mesenchymal stromal or stem cells cultured in basal −MMC media seeded onto type-I collagen networks formed under −MMC, or (I) +MMC conditions (N = 13 +MMC, N = 13 −MMC, p = 0.0001). Scale bars = 25 µm. (J) Average angular standard deviation of actin fibers for H and I. Values are reported as mean ± standard error of measurement. * indicates statistical significance (p<0.05). ** indicates statistical significance (p<0.001).