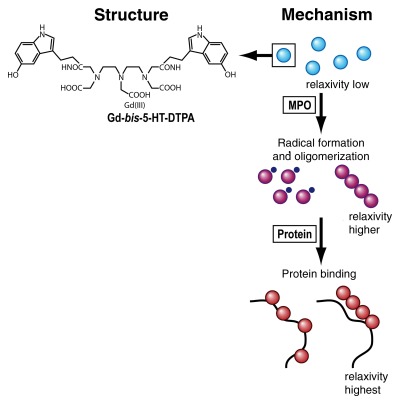
Figure 3:
Chemical structure of activatable MR molecular probe (left) and mechanism of action (right). The probe, gadolinium-5-hydroxytryptamide–diethylene triamine pentaacetic acid (Gd-bis-5-HT-DTPA) has a relatively low relaxivity (low visibility on MR images) in its native state (blue spheres). When the probe reaches an environment rich in myeloperoxidase (MPO), an enzyme secreted by white blood cells in inflammation, its monomers undergo rapid condensation into paramagnetic oligomers (purple spheres), leading to an increase in atomic relaxivity. This change in relaxivity is due to modulation of the rotational correlation time τr. Likewise, the relaxivity and therefore the MR signal increase even further when the probe binds to proteins (red spheres). (Adapted and reprinted, with permission, from reference 11.)