Abstract
Summary
Background and objectives
Hyperuricemia is associated with hypertension, coronary artery disease, and chronic kidney disease. However, there are no specific data on the relationship of uric acid to cardiovascular disease in the chronic hemodialysis setting.
Design, setting, participants, & measurements
Data from 5827 patients on chronic hemodialysis from six countries affiliated with the Dialysis Outcomes and Practice Patterns Study (DOPPS) were analyzed. All laboratory data were based upon the initial cross-section of patients in DOPPS I and II. Cox regression was used to calculate the hazard ratio (HR) of all-cause and cardiovascular (CV) mortality with adjustments for case-mix including 14 classes of comorbidity.
Results
There were no clinically significant differences in baseline characteristics between those who had measured uric acid (n = 4637) and those who did not (n = 1190). Uric acid level was associated with lower all-cause mortality (HR: 0.95, 95% confidence interval [CI]: 0.90 to 1.00 per 1 mg/dl higher uric acid level) and CV mortality (HR: 0.92, 95% CI: 0.86 to 0.99). When analyzed as a dichotomous variable, the adjusted HR at uric acid ≤8.2 mg/dl compared with >8.2 mg/dl was 1.24 (95% CI: 1.03 to 1.49) for all-cause mortality and 1.54 (95% CI: 1.15 to 2.07) for CV mortality.
Conclusions
Higher uric acid levels were associated with lower risk of all-cause and CV mortality in the hemodialysis population. These results are in contrast to the association of hyperuricemia with higher cardiovascular risk in the general population and should be the subject of further research.
Introduction
Epidemiologic studies have demonstrated that ESRD and chronic kidney disease are independent risk factors for cardiovascular events and mortality (1). Moreover, cardiovascular disease is the leading cause of morbidity and mortality (2) in this setting. Elevation of serum uric acid has been shown to be associated with and may be causally related to hypertension, coronary artery disease, and chronic kidney disease (3–5). Hyperuricemia has also been linked to the metabolic syndrome (6,7), and along with the increasing prevalence of obesity and hypertension, uric acid has been more closely scrutinized. A recently published study suggests that in a subset of patients in the Modification of Diet in Renal Disease (MDRD) trial (8) there was an association between hyperuricemia and cardiovascular mortality in patients with chronic kidney disease (9). Only three prior studies have examined uric acid as a predictor of patient outcomes in hemodialysis patients and have suggested a J-shaped relationship between uric acid and mortality. However, each of these studies was based on data collected from a single center with a relatively small sample size (10–12).
We postulated that, similar to the reported associations in the general population, hyperuricemia would be an independent predictor for cardiovascular-related events and death in patients on chronic hemodialysis. However, we were open to the possibility that paradoxical associations could be found, as suggested by prior literature as well as published data with respect to other similar associations often encountered in this patient population (13–15). We investigated this topic in the international Dialysis Outcomes and Practice Patterns Study (DOPPS), which has collected uric acid levels from a substantial number of patients who have undergone uric acid testing as part of their usual care on hemodialysis, and where this issue could be examined in large numbers of patients from multiple dialysis facilities around the world.
Materials and Methods
Data Source
A total of 5827 patients on chronic hemodialysis were available for these analyses from the DOPPS, an ongoing, international, prospective, cohort study of hemodialysis patients and practices (16). The main purpose of the DOPPS is to identify dialysis practices that are associated with improved patient outcomes. In DOPPS I (1996–2001), five European countries (France, Germany, Italy, Spain, and the United Kingdom), Japan, and the United States were included. DOPPS II (2002–2004) included these countries, and added Belgium, Sweden, Canada, Australia, and New Zealand. The DOPPS sampling plan and study methods have been described elsewhere (17,18). Dialysis facilities were randomly selected in each country and only those facilities with at least 20 to 40 patients were included. Patients were then randomly selected within each facility for enrollment in the DOPPS. The DOPPS received local and national institutional review board approval as required. All patients enrolled were at least 18 years old and the subset of patients used for analysis had been on hemodialysis for >3 months.
Availability of Serum Uric Acid Values in DOPPS
The predialysis serum uric acid values were obtained from both DOPPS I and DOPPS II. Study baseline values were used as a representative of each patient's mean serum uric acid level. Among patients with at least three measurements recorded every 4 months, the coefficient of variation was <20%, an acceptable indicator of stability, in 86% of patients. Patients receiving uric acid lowering agents such as allopurinol were included in the analysis (n = 598). Testing practices for uric acid varied widely by country. For example, over 90% of patients in Spain and Japan were tested for uric acid, compared with <10% in the United States. To account for this variability between countries, the study restricted the data analysis to DOPPS countries that performed testing for uric acid on at least 50% of their patients. Additionally, patients from Germany were excluded because of excessive “missingness” of many laboratory measures. The data represent the initial prevalent cross-section of patients from 60 dialysis facilities in France, Italy, and Spain in DOPPS I (n = 1503) and from 161 dialysis facilities from France, Italy, Spain, Japan, Sweden, and Belgium in DOPPS II (n = 4324). Among these patients, serum uric acid measurements were available in 1183 patients from 53 facilities in DOPPS I and 3454 patients from 145 facilities in DOPPS II.
Statistical Analyses
Distribution and descriptive summary measures of serum uric acid across phases and countries were examined. Patient characteristics were compared between groups with and without measured serum uric acid. A clustered logistic regression model was used to examine patient characteristics associated with predictive odds of serum uric acid >8.2 mg/dl versus ≤8.2 mg/dl (80th percentile). The model used the generalized estimating equations (GEE) approach to account for clustering at the facility level, assuming a compound symmetry covariance structure (19) and adjusted for DOPPS phase and country of residence. The patient characteristics that were controlled for are listed in Table 1.
Table 1.
Significant predictors for uric acid >8.2 mg/dl (80th percentile)
| Predictors | AOR | 95% CI | P |
|---|---|---|---|
| Belgium (versus Japan) | 0.29 | (0.17, 0.50) | <0.001 |
| France (versus Japan) | 0.21 | (0.13, 0.33) | <0.001 |
| Italy (versus Japan) | 0.12 | (0.07, 0.20) | <0.001 |
| Spain (versus Japan) | 0.16 | (0.10, 0.25) | <0.001 |
| Sweden (versus Japan) | 0.14 | (0.08, 0.24) | <0.001 |
| Age (per 10 years older) | 0.92 | (0.85, 0.99) | 0.04 |
| Body mass index (per 5 units) | 1.16 | (1.04, 1.29) | 0.008 |
| Residual renal function (yes/no) | 1.24 | (1.00, 1.53) | 0.05 |
| Use of allopurinol (yes/no) | 0.28 | (0.20, 0.39) | <0.001 |
| Use of diuretics (yes/no) | 1.34 | (1.04, 1.72) | 0.02 |
| Albumin-corrected calcium (per 1 mg/dl) | 0.92 | (0.84, 0.99) | 0.03 |
| Serum phosphorus (per 1 mg/dl) | 1.06 | (1.01, 1.12) | 0.02 |
| nPCR (per 0.1 g/kg per day) | 1.18 | (1.13, 1.24) | <0.001 |
| Serum creatinine (per 1 mg/dl) | 1.11 | (1.06, 1.17) | <0.001 |
| Serum potassium (per 1 mEq/L) | 0.89 | (0.80, 1.00) | 0.05 |
| Diabetes | 0.78 | (0.62, 0.99) | 0.04 |
Adjusted for age, black race, male, vintage, body mass index, smoking status, vascular access type, cachexia, residual renal function, use of allopurinol, use of diuretic, use of aspirin, serum LDL, albumin-corrected calcium, serum albumin, serum phosphorus, single-pool Kt/V, white blood cell count, neutrophil/lymphocyte ratio, nPCR, serum ferritin, serum creatinine, serum sodium, serum potassium, 14 summary comorbid conditions, time since ESRD, study phase, country, and accounted for facility clustering effects. AOR, adjusted odds ratio; CI, confidence interval; nPCR, normalized protein catabolic rate.
Outcomes
The primary outcome measure was all-cause mortality, and the secondary outcome was cardiovascular mortality. Stratified Cox proportional hazards regression was used to examine the relationship between all-cause mortality and serum uric acid concentration, controlling for age, black race, sex, body mass index, years with ESRD, albumin-corrected serum calcium, albumin, ferritin, creatinine, phosphorus, use of allopurinol, and 14 summary comorbid conditions: coronary artery disease, cancer (nonskin), other cardiovascular diseases, cerebrovascular disease, congestive heart failure, diabetes, gastrointestinal bleed, HIV/AIDS, hypertension, lung disease, neurologic disease, psychiatric disorder, peripheral vascular disease, and recurrent cellulitis. Normalized protein catabolic rate (nPCR) was adjusted for in a sensitivity analysis, but not included in the model because of excessive missingness (31% of sample). The model was stratified by study phase and country and accounted for facility clustering effects by using the sandwich estimator for the standard errors. The relationship between cardiovascular mortality and serum uric acid concentration was similarly examined by using a Cox proportional hazards regression model with the same adjustments. Cardiovascular events contributing to cardiovascular mortality included: acute myocardial infarction, atherosclerotic heart disease, cardiomyopathy, cardiac arrhythmia, cardiac arrest, congestive heart failure, cerebrovascular accident including intracranial hemorrhage, ischemic brain damage, and anoxic encephalopathy.
There was a varying degree of missingness across different laboratory measurements used in the analysis. A multiple imputation method was carried out to impute these measurements. Five imputed data sets were generated using the sequential regression approach in IVEWARE (20), a stand-alone and SAS-compatible software program for analyses with missing data, developed and maintained at the University of Michigan. The five sets of estimates were pooled by using a simple average, whereas the standard error was estimated using Rubin's formula (21). Among nonimputed confounders in the Cox models, only body mass index (BMI) had a nontrivial amount of missing data (3%). Rather than use a missing indicator variable or complete case analysis, a response propensity approach was used where the cases were weighted by the inverse probability of nonmissing BMI, a confounder for the relationship between serum uric acid and mortality (22). The probability was calculated using a logistic regression model modeling the likelihood of nonmissingness of BMI with the demographic information, laboratory values, and comorbidities serving as independent variables. The statistical analyses were performed using IVEWARE and the SAS statistical package, version 9.1 (SAS Institute, Cary, NC) (19).
Results
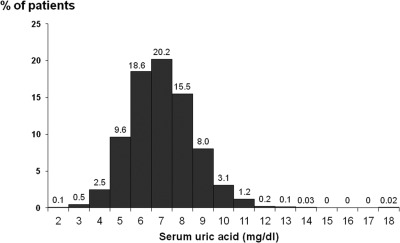
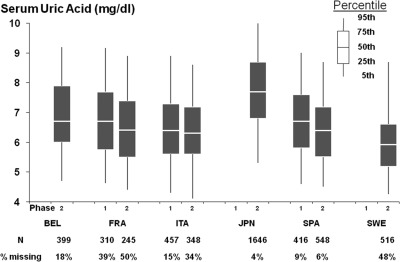
A total of 4637 patients had baseline uric acid concentrations recorded, whereas 1190 did not. There were no clinically significant differences in baseline characteristics between those who had measured uric acid and those who did not (Table 2). Figure 1 reveals baseline serum uric acid is approximately normally distributed with a mean of 6.97 mg/dl. Uric acid distribution by DOPPS phase and country is shown in Figure 2, along with the percentage of patients without uric acid measured.
Table 2.
Patient characteristics by region and uric acid concentration
| Patient Characteristics (mean or %) | Europe |
Japan |
||||
|---|---|---|---|---|---|---|
| Uric Acid >8.2 mg/dl (n = 349) | Uric Acid ≤8.2 mg/dl (n = 2642) | Uric Acid unavailable (Unavailable = 1117) | Uric Acid >8.2 mg/dl (n = 571) | Uric Acid ≤8.2 mg/dl (n = 1075) | Uric Acid unavailable (Unavailable = 73) | |
| Age (years) (mean) | 57.6 ± 15.7 | 63.5 ± 14.4 | 63.5 ± 14.9 | 59.5 ± 12.1 | 61.8 ± 13.7 | 61.0 ± 10.3 |
| Body mass index (mean) | 24.5 ± 4.6 | 24.1 ± 4.5 | 24.3 ± 4.7 | 20.8 ± 3.2 | 20.4 ± 3.0 | 20.2 ± 2.5 |
| Male (%) | 63.9 | 56.8 | 57.2 | 63.2 | 57.5 | 58.9 |
| Vintage (mean) | 5.6 ± 5.9 | 5.3 ± 5.7 | 5.7 ± 6.4 | 7.6 ± 7.2 | 7.1 ± 6.6 | 8.8 ± 8.2 |
| Serum LDL cholesterol (mg/dl) (mean) | 106.0 ± 40.5 | 100.6 ± 38.5 | 96.4 ± 35.7 | 92.7 ± 31.1 | 93.5 ± 29.7 | 94.0 ± 28.7 |
| Albumin-corrected calcium (mg/dl) (mean) | 9.7 ± 1.0 | 9.7 ± 0.9 | 9.7 ± 1.0 | 9.4 ± 0.9 | 9.5 ± 1.0 | 9.2 ± 0.8 |
| Serum phosphorus (mg/dl) (mean) | 5.7 ± 1.9 | 5.3 ± 1.7 | 5.4 ± 1.9 | 6.0 ± 1.6 | 5.5 ± 1.5 | 5.3 ± 1.3 |
| Serum albumin (g/dl) (mean) | 3.9 ± 0.5 | 3.8 ± 0.5 | 3.7 ± 0.5 | 3.9 ± 0.4 | 3.8 ± 0.4 | 3.5 ± 0.4 |
| White blood cell count (103 cells per mm3) (mean) | 7.1 ± 2.2 | 6.9 ± 2.2 | 7.0 ± 2.3 | 6.1 ± 2.0 | 5.9 ± 1.8 | 6.2 ± 1.9 |
| Serum creatinine (mg/dl) (mean) | 10.2 ± 2.9 | 9.0 ± 2.5 | 9.0 ± 2.5 | 11.9 ± 2.8 | 10.7 ± 2.9 | 11.1 ± 2.4 |
| Single-pool Kt/V (mean) | 1.38 ± 0.33 | 1.41 ± 0.31 | 1.45 ± 0.30 | 1.34 ± 0.26 | 1.34 ± 0.29 | 1.48 ± 0.28 |
| nPCR (g/kg per day) (mean) | 1.19 ± 0.27 | 1.09 ± 0.26 | 1.12 ± 0.27 | 1.11 ± 0.22 | 1.00 ± 0.22 | 1.05 ± 0.16 |
| Vascular access | ||||||
| AV fistula (%) | 79.3 | 78.2 | 74.4 | 90.7 | 90.6 | 95.3 |
| graft (%) | 7.1 | 8.5 | 10.7 | 6.5 | 6.7 | 3.1 |
| catheter (%) | 13.3 | 12.4 | 14.3 | 0.9 | 1.4 | 0.0 |
| Residual renal function (%) | 35.8 | 29.0 | 29.4 | 16.6 | 17.9 | 23.1 |
| Comorbidities | ||||||
| coronary heart disease (%) | 32.4 | 33.4 | 42.9 | 24.5 | 25.5 | 26.0 |
| congestive heart failure (%) | 25.8 | 25.0 | 26.5 | 14.6 | 16.4 | 20.3 |
| cerebrovascular disease (%) | 12.7 | 16.8 | 17.9 | 12.3 | 15.9 | 6.3 |
| diabetes (%) | 19.5 | 23.1 | 21.4 | 22.5 | 30.5 | 23.4 |
| Use of allopurinol (%) | 5.2 | 5.9 | 2.1 | 10.3 | 33.0 | 7.9 |
| Use of diuretics (%) | 22.7 | 15.7 | 26.8 | 19.6 | 21.2 | 17.5 |
Based on the initial prevalent cross section of 5827 patients with duration of ESRD >3 months in DOPPS (Dialysis Outcomes and Practice Patterns Study) I (1996–2001) and II (2002–2004). nPCR, normalized protein catabolic rate; AV, arteriovenous.
Figure 1.
Distribution of serum uric acid concentrations.
Figure 2.
Distribution of serum uric acid, by DOPPS country and phase.
Table 1 reveals significant predictors for uric acid concentration >8.2 mg/dl. The adjusted odds ratio of having high uric acid was significantly lower among patients from countries other than Japan. High uric acid was associated with younger age, higher BMI, residual renal function, use of diuretics, higher serum phosphorus, higher serum creatinine, lower serum calcium (albumin corrected), lower serum potassium, and higher nPCR and inversely associated with diabetes and use of allopurinol.
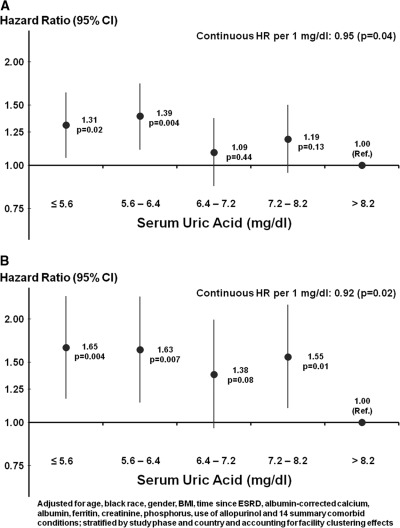
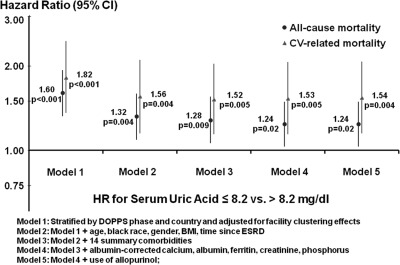
Median follow-up was 23 months with a maximum of 37 months. Uric acid level was associated with lower all-cause mortality (hazard ratio [HR]: 0.95, 95% confidence interval [CI]: 0.90 to 1.00, per 1 mg/dl higher uric acid). As shown in Figure 3A, when examining the association of quintiles of uric acid to mortality, the adjusted hazard ratio for all-cause mortality was statistically significant below a concentration of 6.4 mg/dl. When analyzed as a dichotomous variable, the adjusted HR at concentrations ≤8.2 mg/dl was 1.24 (95% CI: 1.03 to 1.49) compared with >8.2 mg/dl. A sensitivity analysis excluding Japan revealed similar results (HR = 1.21, 95% CI: 0.99 to 1.49). Figure 3B shows that higher uric acid was associated with lower cardiovascular mortality (HR: 0.92, 95% CI: 0.86 to 0.99, per 1 mg/dl higher uric acid). The adjusted HR at concentrations ≤8.2 mg/dl was 1.54 (95% CI: 1.15 to 2.07) compared with >8.2 mg/dl. A sensitivity analysis excluding Japan revealed similar results (HR = 1.68, 95% CI: 1.14 to 2.46). The strength of the association was attenuated by adjusting for patient demographics, whereas adjustments for comorbidities, laboratory values, and allopurinol use had minimal effect (Figure 4). A sensitivity analysis restricted to patients with nonmissing nPCR showed that the HR decreased from 1.28 (95% CI: 1.01 to 1.61) to 1.24 (95% CI: 0.98 to 1.56) after additionally adjusting for nPCR. Mortality model results using the inverse probability weighting of nonmissing BMI were consistent with the complete-case analyses.
Figure 3.
Quintiles of serum uric acid. (A) All-cause mortality and (B) cardiovascular mortality. CI, confidence interval; HR, hazard ratio; Ref., reference.
Figure 4.
Serum uric acid and mortality—levels of adjustment. BMI, body mass index; CI, confidence interval; CV, cardiovascular; DOPPS, Dialysis Outcomes and Practice Patterns Study; HR, hazard ratio.
Discussion
The results of this study indicate that higher uric acid concentrations are associated with lower all-cause and cardiovascular mortality among hemodialysis patients. These results are in contrast to published literature in the general population and raise the possibility that higher uric acid concentrations may be cardioprotective in dialysis patients.
Traditionally, hyperuricemia has been associated with hypertension and chronic kidney disease (3–5). In the Losartan Intervention For Endpoint reduction (LIFE) (23) trial, the losartan arm was associated with regression of left ventricular hypertrophy, a lower incidence of strokes and coronary events, and lower serum uric acid (P < 0.001). One potential mechanism for these results was the uricosuric action of losartan. More recently, Madero et al. made a subset analysis of 839 patients enrolled in the Modification Diet of Renal Disease (MDRD) trial (8). They included patients with measured serum uric acid concentrations and those with chronic kidney disease stages III and IV. The conclusion of the study was that higher uric acid was associated with higher all-cause and cardiovascular-related mortality.
However, neither the LIFE nor the MDRD subset analysis included hemodialysis patients. The results of this study contradict current thinking regarding the linkage of serum uric acid as an independent risk factor for death and cardiovascular events.
Thus far, three prior single-center studies have investigated the relationship of uric acid to all-cause mortality in hemodialysis patients (10–12). None specifically examined cardiovascular, cause-specific mortality. All three studies were characterized by sample sizes of <300 patients and two of the three studies restricted their analyses to patients initiating hemodialysis only. We did not find a J-shaped relationship, as suggested by these earlier studies. There was no upward trend in mortality risk even among patients with uric acid >8.2 mg/dl (the 80th percentile cutoff value). However, we do confirm the previously reported higher risk at lower uric acid values. Even relatively “normal” values of uric acid were associated with higher risk of both death from all causes and cardiovascular disease in our study.
This paradoxical association represents yet another example of the so-called “reverse epidemiology” in the dialysis population and the association remained robust despite several levels of statistical adjustment, albeit the possibility that residual confounding cannot be ruled out. Our study suggests a cardioprotective role for uric acid in the hemodialysis population. An example of the failure of what are normally considered cardioprotective therapies is that the 4D (24) study revealed no mortality benefit from lipid-lowering therapy. In addition, paradoxical associations with mortality have been well described among hemodialysis patients with respect to BP, as well as obesity and higher BMI and LDL cholesterol (13–15,25). The potential mechanisms of this association in hemodialysis patients have yet to be elucidated.
We speculate that higher uric acid among hemodialysis patients is a surrogate for better nutritional status, as evidenced by the positive association with higher nPCR, creatinine, phosphorous, and BMI. However, persistence of the association despite adjustment for these variables, as well as serum albumin, raises the possibility of other potential mechanisms for this apparent protective effect. Other examples analogous to the paradoxical association with uric acid are homocysteine (26) and leptin (27,28). Similar to uric acid, homocysteine is also a marker of better nutritional status and higher protein intake.
Furthermore, a considerable body of literature has focused on inflammation as being causally related to morbidity and mortality in dialysis patients. Uric acid has been shown to have antioxidant properties in vitro (29) and this property may account for some of the benefit observed in the hemodialysis population, where oxidative stress is thought to be mechanistically linked with the excess cardiovascular mortality (30–34). In another study, uric acid was positively correlated with high sensitivity C-reactive protein levels in patients with chronic kidney disease (9). Although the link between uric acid and inflammation has not been entirely clarified, all studies in nondialyzed patients with chronic kidney disease demonstrate better outcomes at lower uric acid concentrations.
The strengths of our study include a large, diverse, and international population of hemodialysis patients in the DOPPS. The fact that detailed information was collected with respect to patient characteristics, comorbidities, and laboratory data permitted comprehensive statistical adjustments to minimize bias inherent in observational studies where associations do not prove causality, and residual confounding cannot entirely be excluded.
Certain other limitations specific to this work are as follows: single, only baseline, and not repeated measurements of uric acid were considered as the independent variable and the assessment of nutritional status at baseline was not comprehensively defined a priori. Furthermore, the effect of allopurinol on survival could not be investigated as only a small number of patients were receiving this therapy on hemodialysis. We found no systematic differences between those who had available uric acid concentrations versus those who did not, suggesting that measurement of uric acid, for the most part, is likely to be a practice pattern at the dialysis facility level, rather than being dictated by specific patient-level clinical indication. Therefore, we expect that the measured association holds in countries that were excluded from this analysis because of a low proportion of patients with measured uric acid concentrations such as the United States, United Kingdom, and Canada.
In conclusion, our study explored the relationship between serum uric acid and both all-cause and cardiovascular mortality and found that higher uric acid concentrations were associated with lower mortality among hemodialysis patients. This unexpected association may, in part, be explained by the fact that higher uric acid concentration among the hemodialysis population is a marker for better nutritional status as evidenced by its association with higher protein intake, serum creatinine, and BMI. The association remained robust despite adjustment for these variables, as well as other markers of nutrition and inflammation including serum albumin and ferritin. Alternative mechanisms, such as uric acid's antioxidant properties and the role of allopurinol in hemodialysis patients, should be the subject of future investigations.
Disclosures
None.
Acknowledgments
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Genzyme (since 2009), and Abbott (since 2009), without restrictions on publications.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS, HEMO Study Group: Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Johnson RJ, Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M: Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41: 1183–1190, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Jonasson T, Ohlin AK, Gottsäter A, Hultberg B, Ohlin H: Plasma homocysteine and markers for oxidative stress and inflammation in patients with coronary artery disease - a prospective randomized study of vitamin supplementation. Clin Chem Lab Med 43: 628–634, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Niskanen LK, Laaksonen DE, Nyyssönen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT: Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: A prospective cohort study. Arch Intern Med 164: 1546–1551, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Ford ES, Li C, Cook S, Choi HK: Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115: 2526–2532, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Choi HK, Ford ES: Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 120: 442–447, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Madero M: High levels of uric acid linked to CKD death risk. Renal Urol News July 11, 2008 [Google Scholar]
- 9. Caravaca F, Martin MV, Barroso S, Cancho B, Arrobas M, Luna E, Sánchez-Casado E: Serum uric acid and C-reactive protein levels in patients with chronic kidney disease. Nefrologia 25: 645–654, 2005 [PubMed] [Google Scholar]
- 10. Suliman ME, Johnson RJ, García-López E, Qureshi AR, Molinaei H, Carrero JJ, Heimbürger O, Bárány P, Axelsson J, Lindholm B, Stenvinkel P: J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis 48: 761–771, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Lee SM, Lee AL, Winters TJ, Tam E, Jaleel M, Stenvinkel P, Johnson RJ: Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am J Nephrol 29: 79–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu SP, Pai ME, Peng YS, Chiang CK, Ho TI, Hung KY: Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in haemodialysis patients. Nephrol Dial Transplant 19: 457–462, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB: Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 351: 2599–2610, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Kalantar-Zadeh K, Kilpatrick R, McAllister C, Greenland S, Kopple JD: Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: The 58th annual fall conference and scientific sessions. Hypertension 45: 811, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kalantar-Zadeh K, Abbott K, Salahudeen A, Kilpatrick RD, Horwich TB: Survival advantages of obesity in dialysis patients. Am J Clin Nutrition 81: 543–554, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Dialysis Outcomes and Practice Patterns Study. Available at: http://www.dopps.org Accessed August 9, 2011
- 17. Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int 57 [suppl 74]: S74–S81, 2000 [Google Scholar]
- 18. Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner M, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis 44 [Suppl 2]: S7–S15, 2004 [DOI] [PubMed] [Google Scholar]
- 19. SAS Institute, Inc SAS/STAT user's guide, Version 8, Volume 2, SAS Institute, Cary, NC, 2000, p 1452 [Google Scholar]
- 20. Raghunathan TE, Lepkowski JM, van Hoewyk M, Solenberger PW: A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodol 27: 85–95, 2001. For associated IVEware software, see http://www.isr.umich.edu/src/smp/ive [Google Scholar]
- 21. Little RJA, Rubin DB: Statistical analysis with missing data, Hoboken NJ, John Wiley & Sons, 2002 [Google Scholar]
- 22. Little RJA, Rubin DB: Statistical analysis with missing data, 2nd Ed. Hoboken, NJ, John Wiley & Sons, 2002 [Google Scholar]
- 23. Høieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Chen C, Dahlöf B; LIFE Study Group: The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int 65: 1041–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Wanner C, Krane V, Rug G, Marz W, Ritz E: Rationale and design of a trial improving outcome of type 2 diabetics on hemodialysis. Die Deutsche Diabetes Dialyse Studie Investigators. Kidney Int Suppl 71: S222–S226, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Suliman M, Stenvinkel P, Qureshi AR, Kalantar-Zadeh K, Barany P, Heimburger O, Vonesh EF, Lindholm B: The reverse epidemiology of plasma total homocysteine as a mortality risk factor is related to the impact of wasting and inflammation. Nephrol Dial Transplant 22: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Scholze A, Rattensperger D, Zidek W, Tepel M: Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity 15: 1617–1622, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Scholze A, Tepel M: Role of leptin in reverse epidemiology in chronic kidney disease. Semin Dial 20: 534–538, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki T, Nishigawara O: Nitrosation of uric acid induced by nitric oxide under aerobic conditions. Nitric Oxide 16: 266–273, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Cappuccio FP, Strazzullo P, Farinaro E, Trevisan M: Uric acid metabolism and tubular sodium handling: Results from a population-based study. JAMA 270: 354–359, 1993 [PubMed] [Google Scholar]
- 31. Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG: Serum uric acid in essential hypertension: An indicator of renal vascular involvement. Ann Intern Med 93: 817–821, 1980 [DOI] [PubMed] [Google Scholar]
- 32. Quiñones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E: Effect of insulin on uric acid excretion in humans. Am J Physiol 268: E1–E5, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Heistad DD, Wakisaka Y, Miller J, Chu Y, Pena-Silva R: Novel aspects of oxidative stress in cardiovascular diseases. Circ J 73: 201–207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madamanchi NR, Hakim ZS, Runge MS: Oxidative stress in atherogenesis and arterial thrombosis: The disconnect between cellular studies and clinical outcomes. J Thromb Haemost 3: 254–267, 2005 [DOI] [PubMed] [Google Scholar]