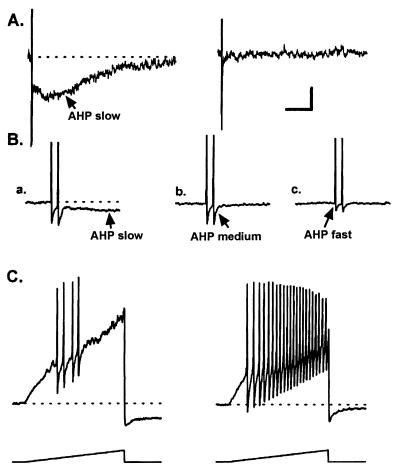
Figure 4.
Effects of BAPTA on the AHPslow and on the excitability of an acutely dissociated rabbit nodose neuron. (A) Bath-applied BAPTA/acetomethylester (10 μM) blocks the AHPslow within 5 min without changing the resting membrane potential or membrane input resistance. APs were evoked by transmembrane depolarizing current pulses (4 nA, 1.5 ms, 10 Hz) and are truncated. (B) Responses recorded at a faster sweep speed to illustrate the kinetics of the AHPfast and AHPmedium, which precede the AHPslow. The AHPfast is unaffected by 10 μM BAPTA/acetomethylester (compare a with b). The Ca2+ dependence of the AHPmedium is illustrated in c, where the neuron is superfused with 100 μM CdCl2 for 30 s, which blocks most of the AHPmedium. The residual component of the AHP recorded in CdCl2 is the AHPfast, which is mediated by delayed rectifier K+ channels. (C) Depression of the AHPslow markedly increases neuronal excitability. The average AP firing frequency induced by a current ramp protocol (1 nA, 2 s) increased from 1 to 5.5 Hz when the AHPslow was blocked. Similar loss of spike-frequency adaptation was observed with bradykinin, prostaglandin D2, histamine, and other inflammatory autacoids (see Table 2). The scale bar represents 3 mV, 2 s in A; 15 mV, 0.25 s in B; and 15 mV, 0.5 s in C. The dashed line represents the resting membrane potential (−60 mV). Resting membrane input resistance was 70 MΩ. Data is from ref. 19 with permission from the American Physiological Society.