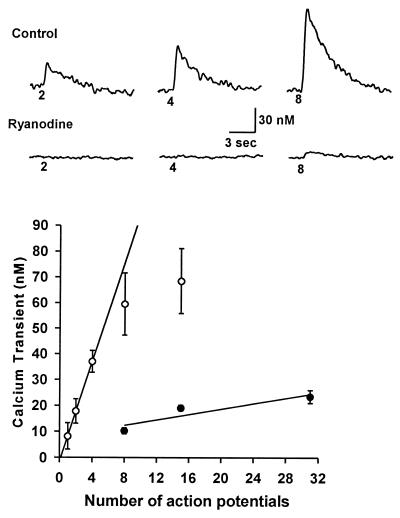
Figure 6.
(Upper) Effect of RY on AP-induced Ca2+ transients. Traces are Ca2+ transients evoked by varying numbers of APs, as indicated below each trace. In control neurons, distinct Ca2+ transients can be elicited by very few APs. In contrast, in the presence of 10 μM RY, a CICR inhibitor, at least eight APs are required to generate a discernible change in [Ca2+]i. Suppression of the Ca2+ transient by RY is due to its effect on CICR and not the result of nonspecific effects on Ca2+ channels; the kinetics and amplitude of ICa elicited by APs are completely unaffected by RY. (Lower) Effect of RY on the relation between the amplitude of Ca2+ transients and number of APs. ○ and ● are mean amplitudes of Ca2+ transients evoked by varying numbers of action potentials for control (n = 10) and for RY-treated nodose neurons (n = 3), respectively. Linear regression of data from control (≤4 action potentials) and RY-treated cells yielded slopes of 9.6 ± 0.01 and 0.5 ± 0.23 nM per AP, respectively. Comparison of the slopes illustrates that CICR is capable of amplifying the “trigger” Ca2+ resulting from AP-induced Ca2+ influx by 20-fold. Data is modified from ref. 16 with permission from Journal of Physiology (London).