Table 4.
The detail of clinical treatment course of two patients (cases 11 and 12) who had tuberculomas.
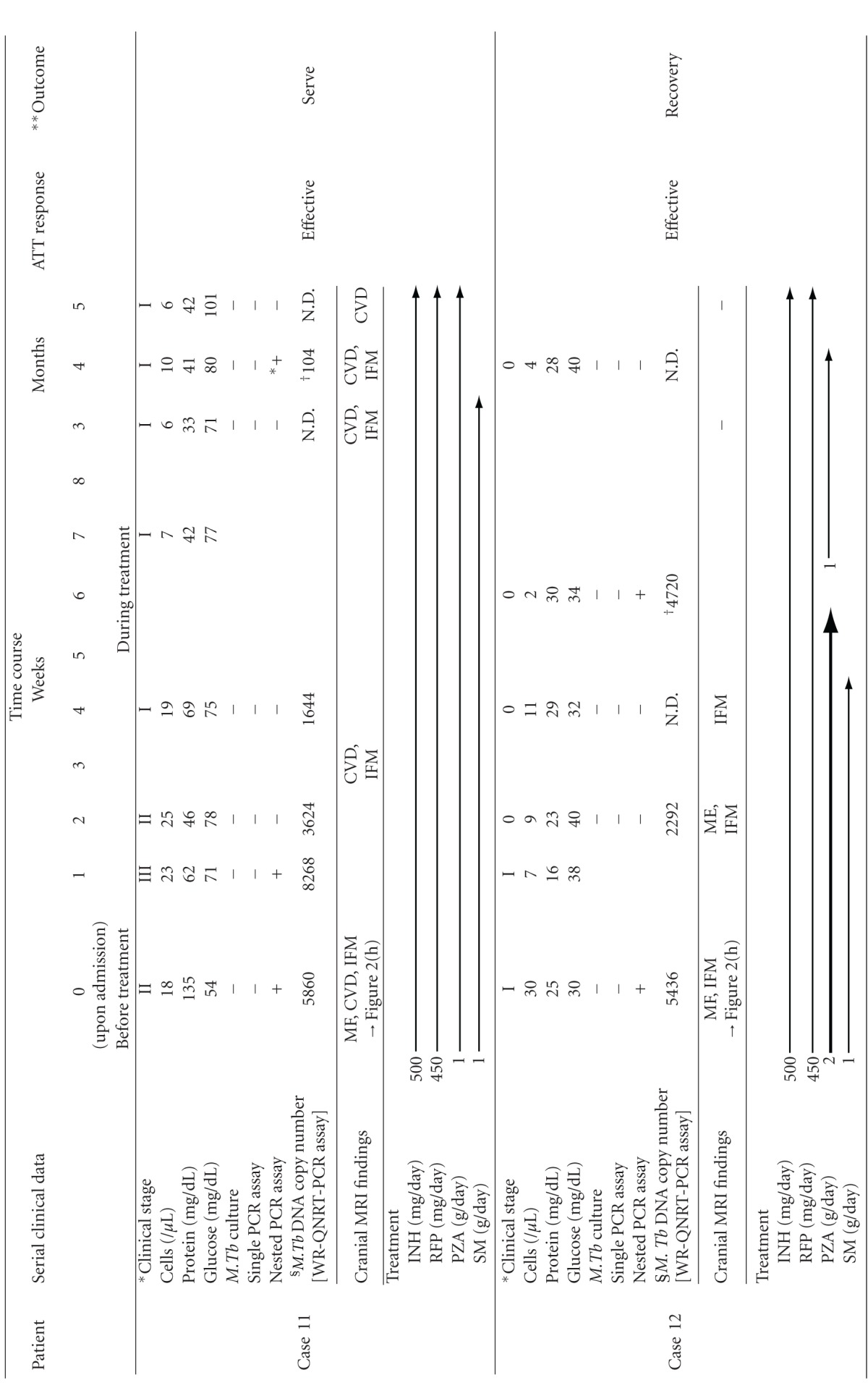
|
CSF: cerebrospinal fluid, PCR: polymerase chain reaction, M. Tb: Mycobacterium tuberculosis, +: positive, ∗+: slightly positive, −: negative, §: per 1 ml CSF, MF: meningeal enhancement, CVD: cerebrovascular disorder, IFM: intracranial focal mass, INH: isoniazid, RFP: rifampicin, PZA: pyrazinamide, SM: streptomycin sulfate; †: transiently positive result.
*According to the clinical stages defined by the British Medical Research Council: stage 0: no define neurologic symptoms, stage I: slight signs of meningeal irritation with slight clouding of consciousness, stage II: moderate signs of meningeal irritation with moderate disturbance of consciousness and neurologic defects, stage III: severe disturbance of consciousness and neurologic defects. **Outcome classified as recovery with minor or no neurologic impairment, severe neurologic impairment, and death.
