Figure 7.
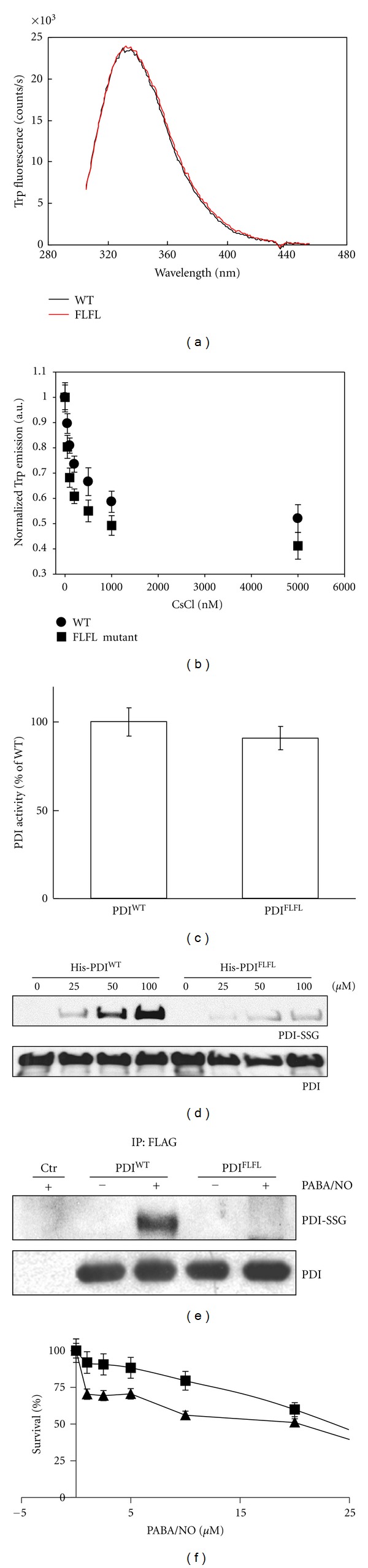
Mutations impair PABA/NO-induced S-glutathionylation of PDI. (a) Spectroscopic analysis of PDI-WT (black) and PDI-FLFL (red) was performed using trypophanyl fluorescence of the purified proteins. (b) Quenching experiments were performed to further assess protein folding for PDI-WT (●) and PDI-FLFL (■). (c) The enzymatic activity of PDI-WT and PDI-FLFL was evaluated using the insulin turbidity assay. No statistical differences were observed in the folding (a-b) or isomerase activity (c), P > 0.05. (d) S-glutathionylation of PDI-WT and PDI-FLFL was evaluated following drug treatment. (e) MCF7 cells overexpressing PDI-WT or PDI-FLFL were treated with DMSO or 20 μM PABA/NO. Following immunoprecipitation, the samples were evaluated by Western blot with anti-S-glutathionylation and anti-PDI antibodies. (f) MCF7 cells transfected with control (▲) or PDI-FLFL (■) were seeded in a 96-well plate and treated with 0–25 μM PABA/NO. Cell viability was measured at 72 h with the MTT assay [14]. Data represent the mean for 3 independent experiments ± S.D.
