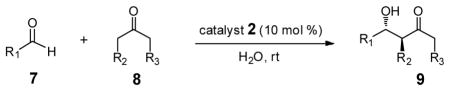
Table 2.
Representative PQS-proline (2)-Catalyzed Reactionsa
 | |||||
|---|---|---|---|---|---|
| entry | product | time (h) | yieldb (%) | ant i:sync | eed (%) |
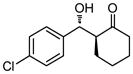
| 1 |
 9a |
30 | 88 | 82:18 | 90 |
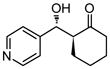
| 2 |
 9b |
18 | 90 | 90:10 | 90 |
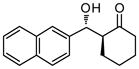
| 3 |
 9c |
48 | 74 | 86:14 | 92 |
| 4 |
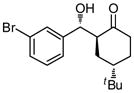 9d |
36 | 80 | 83:17 | 91 |
| 5 |
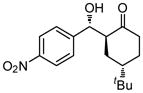 9e |
18 | 85 | 85:15 | 79 |
| 6 |
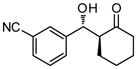 9f |
30 | 80 | 90:10 | 97 |
| 7 |
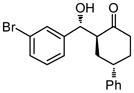 9g |
36 | 82 | 68:32 | 86 |
| 8 |
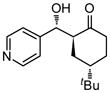 9h |
18 | 85 | 89:11 | 75 |
| 9 |
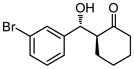 9i |
36 | 82 | 84:16 | 86 |
| 10 |
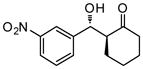 9j |
24 | 90 | 90:10 | 91 |
The reactions were performed with aldehyde (0.1 mmol), ketone (0.5 mmol) and catalyst 2 (0.01 mmol) at rt.
Combined yield of isolated diastereomers.
Determined by 1H NMR of the crude product.
Determined by chiral-phase HPLC analysis for anti-product.
