Figure 1. A model for TA targeting by the GET pathway.
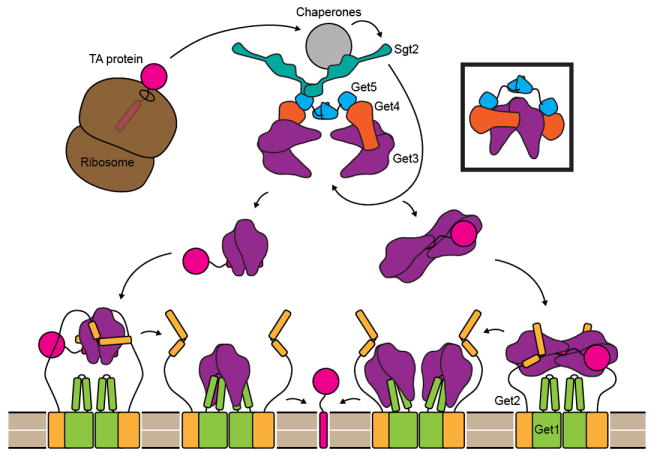
After protein synthesis is complete, a complex consisting of cellular chaperones, two copies each of Get4 and Get5 and at least one dimer of Sgt2 binds the TM helix of TA-proteins. The Get4/Get5 complex recruits Get3, and Sgt2 transfers ER destined TA-proteins to Get3. Each Get4 may bind a separate dimer of Get3, or the same dimer (boxed). The Get3/TA-protein complex may contain two or four copies of Get3. The stoichiometry of the Get1/Get2 complex within the ER membrane is unknown but is shown here as a dimer of 1:1 Get1 to Get2 dimers. Initially Get2 binds the Get3/TA-protein complex and is then displaced by Get1. Get1 binding is coupled to the opening of Get3, leading to release and integration of the TA-protein. In the case of a dimeric Get3/TA complex, Get1 and Get2 could bind the Get3 subunits symmetrically (left pathway). Alternatively, Get2 and Get1 could bind a tetramer of Get3 asymmetrically (right pathway).
