Abstract
Concomitant morphological and molecular analyses have led to major breakthroughs in the taxonomic organization of the phylum Glomeromycota. Fungi in this phylum are known to form arbuscular mycorrhiza, and so far three classes, five orders, 14 families and 29 genera have been described. Sensu lato, spore formation in 10 of the arbuscular mycorrhiza-forming genera is exclusively glomoid, one is gigasporoid, seven are scutellosporoid, four are entrophosporoid, two are acaulosporoid, and one is pacisporoid. Spore bimorphism is found in three genera, and one genus is associated with cyanobacteria. Here we present the current classification developed in several recent publications and provide a summary to facilitate the identification of taxa from genus to class level.
Keywords: Archaeosporomycetes, endomycorrhizas, evolution, Gigasporales, Glomerales, Glomeromycetes, Paraglomeromycetes, phylogeny, VA mycorrhiza
INTRODUCTION
Glomeromycota taxonomy was largely morphologically driven up to the end of the last millennium. All glomeromycotean fungi, except one genus, are known to form arbuscular mycorrhiza. Their identification was based on spore morphology, spore formation, and spore wall structure (e.g. Gerdemann & Trappe 1974, Walker & Sanders 1986, Morton & Benny 1990, Schenck & Pérez 1990). However, as soon as molecular phylogenetic tools became available, they were included in taxonomic analyses (e.g. Simon et al. 1992) and soon became the drivers of the establishment of a new taxonomy (Morton & Redecker 2001, Schüßler et al. 2001). In 1990, without the benefit of molecular aspects, the arbuscular mycorrhiza-forming fungi were organized in three families (Acaulosporaceae, Gigasporaceae, and Glomeraceae) and six genera (Acaulospora, Entrophospora, Gigaspora, Glomus, Sclerocystis, and Scutellospora) within one order, Glomerales (Morton & Benny 1990) of the fungal phylum Zygomycota. That classification was based on spore morphology and spore formation characteristics (acaulosporoid, entrophosporoid, gigasporoid, glomoid, radial-glomoid, and scutellosporoid). Differences in spore wall structure were used at the species level.
Today, we accept three classes (Archaeosporomycetes, Glomeromycetes, and Paraglomeromycetes), five orders (Archaeosporales, Diversisporales, Gigasporales, Glomerales and Paraglomerales), 14 families, 29 genera and approximately 230 species (e.g. Morton & Redecker 2001, Schüßler et al. 2001, Oehl & Sieverding 2004, Walker & Schüßler 2004, Sieverding & Oehl 2006, Spain et al. 2006, Oehl et al. 2008, 2011a–d, Palenzuela et al. 2008).
Until recently, it was unclear whether glomoid and gigasporoid species could be further divided into different morphological groups congruent with the major phylogenetic clades obtained by molecular analyses. A first revision of the sporogenous cell forming (gigasporoid and scutellosporoid) Glomeromycetes according to concomitant morphological and phylogenetic features (Oehl et al. 2008) was not accepted by all mycologists (Morton & Msiska 2010). However, later studies with a broader database (e.g. Goto et al. 2010, 2011, Oehl et al. 2010, 2011b) confirmed that the revised genus Scutellospora, as well as the new Racocetra, Cetraspora, Dentiscutata, and Orbispora, are monophyletic.
A large group of species forms glomoid spores, and it had been believed that there were too few morphological characters of significance to differentiate them. Taxonomists have consequently started basing groupings of the glomoid species almost exclusively on molecular phylogenetic characters. A recent revision of these glomoid species has, however, shown that molecular phylogeny is actually congruent with the morphological characteristics of these fungi (Oehl et al. 2011c). Fungal species with entrophosporoid spore formation were also revised (Oehl et al. 2011d). The objective of this paper is to present the current overall classification system of Glomeromycota that has emerged from these recent studies, and to summarize the major morphological features in the phylum down to genus level.
MATERIALS AND METHODS
The morphological, molecular, and phylogenetic analyses performed are presented in a series of recent publications dealing with different species groups of Glomeromycota (e.g. Oehl et al. 2006, 2010, 2011a, b, d, f, Sieverding & Oehl 2006, Silva et al. 2006, Spain et al. 2006, Palenzuela et al. 2008, 2010, 2011).
RESULTS AND DISCUSSION
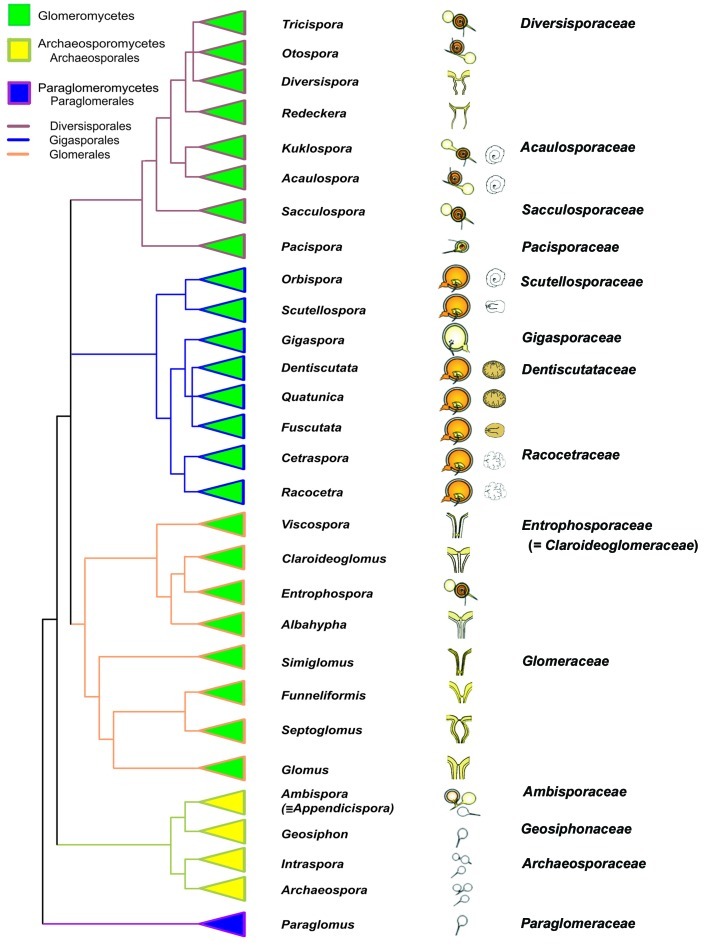
Figure 1 is a schematic tree for Glomeromycota based on molecular phylogenetic analyses of the SSU, ITS region, partial LSU of the rRNA gene, and partial β-tubulin gene (e.g. Oehl et al. 2008, 2010, 2011a–d). In Table 1, the major morphological features of all higher level taxa are presented, with the taxa arranged according to their taxonomic rank down to genus. Three glomeromycotean classes, five orders, 14 families, and 29 genera have been recognized to date (Table 1). Sensu lato, spore formation in 10 of the arbuscular mycorrhiza-forming genera have exclusively glomoid, one has gigasporoid, seven have scutellosporoid, four have entrophosporoid, two genera have acaulosporoid, and one has pacisporoid spore formation, while three genera show spore bimorphism, and one genus is associated with cyanobacteria (the only one not forming arbuscular mycorrhizas).
Fig. 1.
Representative tree of the phylum Glomeromycota based on molecular (SSU, ITS region, partial LSU of the rRNA gene, and partial β-tubuline gene) and morphological analyses (spore wall structures, structures of the spore bases and subtending hyphae, germination, and germination shield structures). Adapted from (Oehl et al. 2008, 2011a–d). The drawings in the central columns show the spore formation types of the genera, and the typical germination shields for those genera which form persistent shields already during spore formation.
Table 1. Major morphological characters for higher level taxa of Glomeromycota from class to genus level.
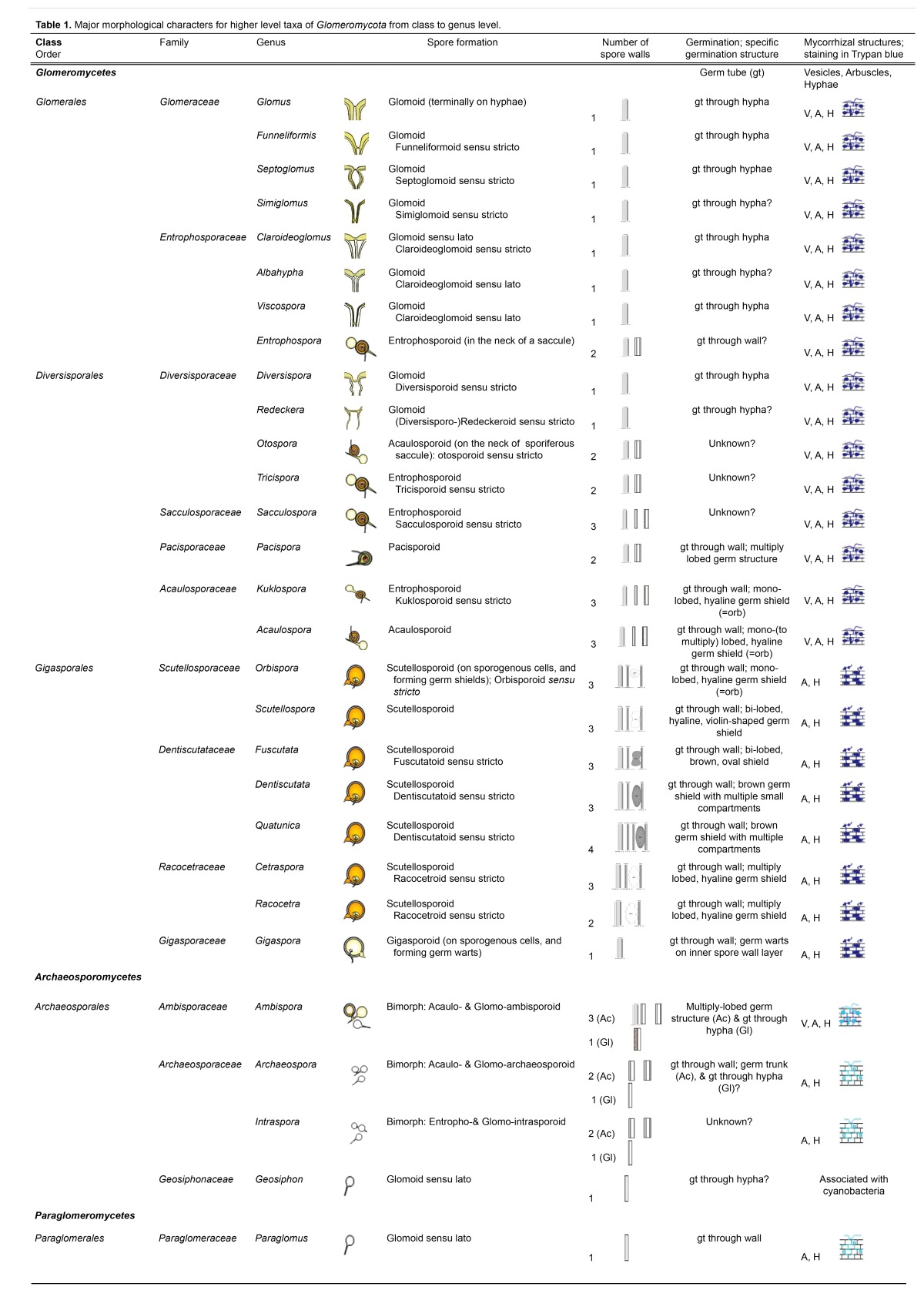 |
Hitherto, Paraglomeromycetes are monogeneric (Table 1), are characterized by mono-walled spores formed terminally on hyphae (i.e. glomoid spores sensu lato), and germinate directly through the spore wall. Their arbuscular mycorrhizal structures do not or only faintly stain in trypan blue. Archaeosporomycetes includes organisms that are exclusively bimorphic since they form either acaulosporoid or entrophosporoid spores simultaneously with glomoid spores, or are associated with cyanobacteria. The mycorrhizal structures of Archaeosporaceae are similar to those of Paraglomeraceae, while Ambisporaceae form vesicular-arbuscular mycorrhizal structures staining pale blue in trypan blue. In contrast, mycorrhizal structures in Glomeromycetes stain blue to dark blue in trypan blue. In Glomeromycetes, Gigasporales species do not form intraradical vesicles but auxiliary cells in soils, which clearly distinguish them from Glomerales and Diversisporales.
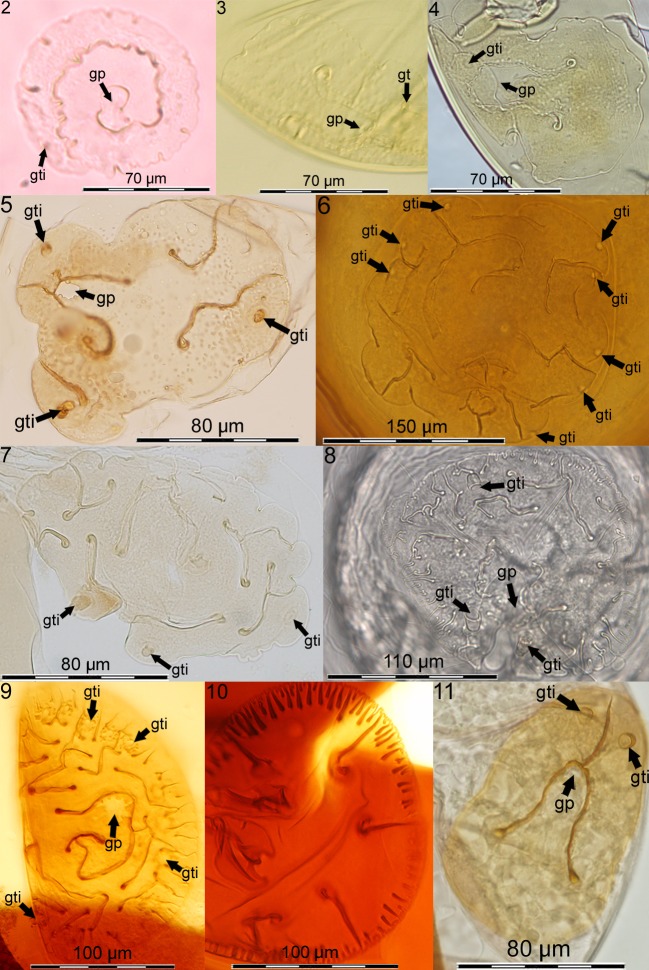
Gigasporales exhibit gigasporoid or scutellosporoid spore formation (Oehl et al. 2011b), i.e. spores formed terminally on sporogenous cells and with either germ warts on the inner surface of the mono-walled spore wall (gigasporoid; Gigasporaceae), or a discrete germination shield on the innermost (= ‘germinal wall’) of 2–4 walls (scutellosporoid). There are three families with scutellosporoid spore formation (sensu lato): Dentiscutataceae, Racocetraceae and Scutellosporaceae (Oehl et al. 2008). Scutellosporaceae form mono-lobed (Orbispora) or bi-lobed (Scutellospora), hyaline germination shields (Figs 2–4). Racocetraceae species form wavy-like, multiply lobed, hyaline germination shields and have either two (Racocetra) or three (Cetraspora) spore walls (Figs 5–8). Dentiscutataceae species form yellow-brown to brown germ shields that are bi-lobed (Fuscutata; Fig. 9) or with multiple compartments (Dentiscutata, triple-walled; Quatunica four-walled; Figs 10–11).
Figs 2–11.
Characteristic germination shields in Gigasporales with germ pore (gp) as connection between spore cell contents and shields that are positioned on the surface of the germinal wall; germ tubes emerge from germ tube initiations (gti). Fig. 2. Orbispora pernambucana (isotype, ZT Myc 641) with mono-lobed, hyaline germ shield (orb). Figs 3–4. Scutellospora calospora (photo taken at INVAM) and S. dipurpurescens (holotype OSC #83343) have bi-lobed, violin-shaped, hyaline shields. Figs 5–8. Racocetra coralloidea (type, OSC #31026), R. castanea (ex type, ZT Myc 4377), Cetraspora nodosa (isotype, DPP, Szczecin, Poland) and C. helvetica (isotype, ZT Myc 3038) have wavy-like, multiply lobed, hyaline shields. Figs 9–11. Dentiscutataceae shields are yellow brown to brown. Fig. 9. Dentiscutata reticulata (photo taken at INVAM) shields with multiple small compartments. Fig. 10. Quatunica erythropa (photo taken at INVAM) is assumed to be the only known species in Glomeromycota with four spore walls. Fig. 11. Fuscutata heterogama (ex type, ZT Myc 642) has a bi-lobed, oval to ovoid shield.
In Archaeosporales and Diversisporales, four genera have spore formation laterally on the neck of terminal or intercalary sporiferous saccules (= acaulosporoid sensu lato; Table 1): Acaulospora, Otospora, and the bi-morphic Ambispora and Archaeospora. These genera can easily be separated on spore wall number and spore wall structure (Palenzuela et al. 2008). Triple-walled Acaulospora species have a characteristic granular, ‘beaded’ inner wall surface (Morton & Benny 1990), which is absent in acaulo-ambisporoid spores of triple-walled Ambispora species (Spain et al. 2006, Palenzuela et al. 2011). The wall structure of the bi-walled Otospora is more complex than that of bi-walled Archaeospora species (Palenzuela et al. 2008).
In Archaeosporales, Diversisporales, and Glomerales, there are five genera with spore formation within the neck of terminal or intercalary sporiferous saccules (i.e. entrophosporoid sensu lato; Table 1): Entrophospora, Kuklospora, Sacculospora, Tricispora, and bimorphic Intraspora (Oehl et al. 2011d). Triple-walled Kuklospora has the characteristic granular, ‘beaded’ inner wall surface of Acaulosporaceae (Sieverding & Oehl 2006), which is absent in spores of triple-walled Sacculospora (Oehl et al. 2011d). The wall structure of bi-walled Entrophospora and Tricispora is more complex than that of bi-walled, bimorphic Intraspora species (Sieverding & Oehl 2006, Oehl et al. 2011d). Entrophospora and Tricispora can be distinguished through the two cicatrices (scars) and pore structures proximal and distal to the sporiferous saccule: the proximal pore is wide in Tricispora and closed by a septum, while it is narrow and closed by a plug in Entrophospora. The distal pore and scar is absent in Entrophospora from the structural layer, and formed only on the overlying, hyaline, evanescent layer, while, in light microscopy, the distal pore with a distal scar is obvious in Tricispora (Sieverding & Oehl 2006, Palenzuela et al. 2010, Oehl et al. 2011d).
In Diversisporales and Glomerales, 10 genera exclusively differentiate mono-walled, glomoid (9) or bi-walled pacisporoid (1) spores, all formed on subtending hyphae (Oehl & Sieverding 2004, Oehl et al. 2011a). The morphological differentiation of the glomoid species is mainly based on the morphology of the subtending hyphae of the spores, and spore wall structure. Spores of Funneliformis, Glomus, Septoglomus, and Simiglomus species have subtending hyphae that are concolorous or slightly lighter in colour than the spore wall (Table 1, Figs 12–16). Albahypha, Claroideoglomus, and Viscospora form spores in which the structural wall layer is continuous with the subtending hyphal wall layer, but the subtending hyphae are hyaline (Figs 17–19). In contrast, Diversispora and Redeckera form spores whose structural wall layer is not obviously continuous with the hyphal wall layer (Figs 20–21); consequently, such spores appear to have included ‘endospores’.
Figs 12–21.
Characteristic spore bases and subtending hyphae (sh) in Glomeromycetes genera with glomoid spore formation. Figs 12–13. Glomus ambisporum (Oehl collection, from Bolivia) and G. aureum (type, ZT Myc 822) with two wall layers (SWL1 and SWL2), marked introverted wall thickening at sb and in sh, and a small, bridging septum (sp). Fig. 14. Funneliformis coronatus (ex type, Oehl collection) with funnel-shaped sh and conspicuous sp; introverted wall thickening is lacking. Fig. 15. Septoglomus constrictum (Oehl collection, from Switzerland) with conspicuous septum that sometimes resembles a plug. Fig. 16. Simiglomus hoi (Oehl collection, specimen mounted at York university) with cylindrical sh; sh wall thickened over long distances; several septae are regularly observed within the hyphae; no introverted wall thickening at sb, pore at sb generally opened. Fig. 17. Claroideoglomus etunicatum (Oehl collection, from Bolivia) with funnel/bill-shaped, white sh; all Entrophosporaceae (syn. Claroideoglomeraceae) with characteristic color change of structural wall layer at sb, if spores are pigmented. Fig. 18. Albahypha drummondii (type, DPP) with slightly funnel-shaped, white sh. Fig. 19. Viscospora viscosa (ex type, photo taken at INVAM) with cylindrical, white hypha; sp within sh in some distance of sb; introverted wall thickening of sh at sp position, here not that obvious as usually found for this species; viscose spore surface. Fig. 20. Diversispora versiformis with short, fragile sh that is principally continuous with semi-persistent outermost spore wall layer (SWL1) but not with structural layer SWL2 (Oehl collection, from Tibet). Fig. 21. Redeckera fulva (Oehl ex Trappe collection) with inflating sh and conspicuous broad sp exactly at spore base.
Funneliformis, Glomus, Septoglomus, and Simiglomus can be separated by the structure of the spore base and subtending hyphae (sh). Glomus species often have an introverted wall thickening (Oehl et al. 2011a; Figs 12–13) which is only otherwise seen in Viscospora. Funneliformis species generally have an easily visible septum in the area of the spore base, and their sh are regularly funnel-shaped to cylindrical (Fig. 14). Septoglomus species have constricted to cylindrical sh, and usually there is a septum at the spore base (Fig. 15). In Simiglomus, sh are cylindrical and thick-walled, and they have several septa some distance from the spore base (Fig. 16). Claroideoglomus has funnel- to bird-bill-shaped sh, with sh and sh walls that are > 2.5 times wider at the spore base than some distance from the base (Fig. 17). Albahypha has slightly funnel to bill-shaped sh and sh walls that are < 2.0 times wider at the spore base than at some distance from the base (Fig. 18), and Viscospora has cylindrical sh (Fig. 19) with an sh wall that may be thickened over large distances and may bear septa in the hyphae with introverted wall thickenings in the area of the septum. In Diversispora, the sh are usually quite fragile and hyaline, distal to the pore closure at the spore base or in the sh (Fig. 20). Redeckera species have a broad septum at the spore base (Fig. 21), and the structural wall layer does not continue more than 5–15 μm into the subtending hypha, and thus, the sh may inflate at this distance from the spore base.
There are three bi-morphic genera with glomoid spore formation. Glomo-ambisporoid spores have a subhyaline to ochraceous, evanescent outer wall layer continuous with the outer acaulo-ambisporoid spore wall, while the second, structural layer is hyaline and continuous with the middle wall of acaulo-ambisporoid spores (Spain et al. 2006, Palenzuela et al. 2011). Glomo-archaeosporoid and Glomo-intrasporoid spores are among the smallest within Glomeromycota (ca. 30 μm), and thus difficult to observe.
PERSPECTIVES
Further separations of genera and families can be expected in the near future since many species and several species groups have not yet been analyzed by molecular phylogenetic methods (e.g. Glomus group Ab1, sensu Oehl et al. 2011a). Major efforts are needed to properly describe the morphology of, in particular, small-spored Glomus species (Błaszkowski et al. 2009a, b, 2010a, b), and it is difficult to predict how morphological identification will develop in those fungi. Other recent progress has been made on Acaulospora species with pitted surface ornamentation, where several species, that superficially all resembled A. scrobiculata, have been separated through extensive morphological and molecular spore analyses (e.g. Oehl et al. 2006, 2011e, f). The establishment of international and national collections of arbuscular mycorrhizal fungi, such as INVAM in Morgantown (International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi, West Virginia State University, USA), CICG in Blumenau (International Collection of Glomeromycota at FURB, Santa Catarina State, Brazil), GINCO-BEL in Louvain-La-Neuve (Glomeromycota In Vitro Collection at the Catholic University of Louvain, Belgium), or SAF in Zurich (Swiss Collection of Arbsucular Mycorrhizal Fungi at Agroscope ART, Switzerland) will facilitate further progresses in the taxonomy of glomeromycotean fungi that were thought to have not enough criteria to morphologically separate them unequivocally into the higher level taxa they phylogenetically belong to. Currently, several arbuscular mycorrhizal fungi are being described as new to science each year by an increasing numbers of research groups. A simple, but well justified conclusion is that, as a result of future concomitant morphological and molecular analyses, yet more higher level taxa will be proposed in this ancient fungal phylum, at all levels from class down to genus.
Acknowledgments
We acknowledge the help of many current and former students and technicians in Switzerland, Spain, and Brazil for outstanding support (especially David Schneider, Robert Bösch, Giacomo Busco, Louis Lawouin, Domingo Alvarez, Danielle Silva, Natália Sousa, and Daniele Magna). This study has been supported within the Swiss National Science Foundation (SNSF) Project 315230_130764/1, by the SNSF Programme NFP48 ‘Landscapes and habitats of the Alps’, by the Spanish Ministry of Environment (MMA-OAPN, project 70/2005) and FAECA (Junta de Andalucía, Spain, Project 92162/11), and by the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) and the UFPE which provided grants to F. Oehl as ‘visiting professor’, and FACEPE which also provided financial support for G.A. Silva.
REFERENCES
- Błaszkowski J, Kovács GM, Balázs TK. (2009a) Glomus perpusillum, a new arbuscular mycorrhizal fungus. Mycologia 101: 247–255 [DOI] [PubMed] [Google Scholar]
- Błaszkowski J, Ryszka P, Oehl F, Koegel S, Wiemken A, et al. (2009b) Glomus achrum and G. bistratum, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Botany 87: 260–271 [Google Scholar]
- Błaszkowski J, Wubet T, Harikumar VS, Ryszka P, Buscot F. (2010a) Glomus indicum, a new arbuscular mycorrhizal fungus. Botany 88: 132–143 [Google Scholar]
- Błaszkowski J, Kovács GM, Balázs TK, Orlowska E, Sadravi M, et al. (2010b) Glomus africanum and G. iranicum, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycologia 102: 1450–1462 [DOI] [PubMed] [Google Scholar]
- Gerdemann JW, Trappe JM. (1974) The Endogonaceae of the Pacific Northwest. Mycologia Memoir 5: 1–76 [Google Scholar]
- Goto BT, Silva GA, Maia LC, Oehl F. (2010) Dentiscutata colliculosa, a new species in the Glomeromycetes from Northeastern Brazil with colliculate spore ornamentation. Nova Hedwigia 90: 383–393 [Google Scholar]
- Goto BT, Silva GA, Maia LC, Souza RG, Coyne D, et al. (2011) Racocetra tropicana, a new species in the Glomeromycetes from tropical areas. Nova Hedwigia 92: 69–82 [Google Scholar]
- Morton JB, Benny GL. (1990) Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon 37: 471–491 [Google Scholar]
- Morton JB, Redecker D. (2001) Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93: 181–195 [Google Scholar]
- Morton JB, Msiska Z. (2010) Phylogenies from genetic and morphological characters do not support a revision of Gigasporaceae (Glomeromycota) into four families and five genera. Mycorrhiza 20: 483–496 [DOI] [PubMed] [Google Scholar]
- Oehl F, Sieverding E. (2004) Pacispora, a new vesicular-arbuscular mycorrhizal fungal genus in the Glomeromycetes. Journal of Applied Botany and Food Quality 78: 72–82 [Google Scholar]
- Oehl F, Sýkorová Z, Redecker D, Wiemken A, Sieverding E. (2006) Acaulospora alpina, a new arbuscular mycorrhizal fungal species characteristic for high mountainous and alpine regions of the Swiss Alps. Mycologia 98: 286–294 [DOI] [PubMed] [Google Scholar]
- Oehl F, Souza FA, Sieverding E. (2008) Revision of Scutellospora and description of five new genera and three new families in the arbuscular mycorrhiza-forming Glomeromycetes. Mycotaxon 106: 311–360 [Google Scholar]
- Oehl F, Jansa J, Souza FA, Silva GA. (2010) Cetraspora helvetica, a new ornamented species in the Glomeromycetes from Swiss agricultural fields. Mycotaxon 114: 71–84 [Google Scholar]
- Oehl F, Silva GA, Goto BT, Sieverding E. (2011a) Glomeromycetes: three new genera and glomoid species reorganized. Mycotaxon 116: 75–120 [Google Scholar]
- Oehl F, Silva DKA, Maia LC, Sousa NMF, Vieira HEE, Silva GA. (2011b) Orbispora gen. nov., ancestral in the Scutellosporaceae (Glomeromycetes). Mycotaxon 116: 161–169 [Google Scholar]
- Oehl F, Silva GA, Goto BT, Maia LC, Sieverding E. (2011c) Glomeromycota: two new classes and a new order. Mycotaxon 116: 365–379 [Google Scholar]
- Oehl F, Silva GA, Sánchez-Castro I, Goto BT, Maia LC, Vieira HEE, Barea JM, Sieverding E, Palenzuela J. (2011d) Revision of Glomeromycetes with entrophosporoid and glomoid spore formation with three new genera. Mycotaxon 117: 297–316 [Google Scholar]
- Oehl F, Sýkorová Z, Błaszkowski J, Sánchez-Castro I, Coyne D, et al. (2011e) Acaulospora sieverdingii, an ecologically diverse new fungus in the Glomeromycota, described from lowland temperate Europe and tropical West Africa. Journal of Applied Botany and Food Quality 84: 47–53 [Google Scholar]
- Oehl F, Silva GA, Palenzuela J, Sánchez-Castro I, Castillo C, Sieverding E. (2011f) Acaulospora punctata, a new fungal species in the Glomeromycetes from mountainous altitudes of the Swiss Alps and Chilean Andes. Nova Hedwigia 93: 353–362 [Google Scholar]
- Palenzuela J, Ferrol N, Boller T, Azcón-Aguilar C, Oehl F. (2008) Otospora bareai, a new fungal species in the Glomeromycetes from a dolomitic shrub-land in the National Park of Sierra de Baza (Granada, Spain). Mycologia 100: 282–291 [DOI] [PubMed] [Google Scholar]
- Palenzuela J, Barea JM, Ferrol N, Azcón-Aguilar C, Oehl F. (2010) Entrophospora nevadensis, a new arbuscular mycorrhizal fungus, from Sierra Nevada National Park (southeastern Spain). Mycologia 102: 624–632 [DOI] [PubMed] [Google Scholar]
- Palenzuela J, Barea JM, Ferrol N, Oehl F. (2011) Ambispora granatensis, a new arbuscular mycorrhizal fungus, associated with Asparagus officinalis in Andalucía (Spain). Mycologia 103: 333–340 [DOI] [PubMed] [Google Scholar]
- Schenck NC, Pérez Y. (1990) Manual for the Identification of VA Mycorrhizal Fungi. 3rd edn: Gainesville, FL: Synergistic Publications; [Google Scholar]
- Schüßler A, Schwarzott D, Walker C. (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research 105: 1413–1421 [Google Scholar]
- Sieverding E, Oehl F. (2006) Revision of Entrophospora and description of Kuklospora and Intraspora, two new genera in the arbuscular mycorrhizal Glomeromycetes. Journal of Applied Botany and Food Quality 80: 69–81 [Google Scholar]
- Silva GA, Lumini E, Maia LC, Bonfante P, Bianciotto V. (2006) Phylogenetic analysis of Glomeromycota by partial LSU rDNA sequences. Mycorrhiza 16: 183–189 [DOI] [PubMed] [Google Scholar]
- Simon L, Lalonde M, Bruns TD. (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Applied and Environmental Microbiology 58: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain JL, Sieverding E, Oehl F. (2006) Appendicispora, a new genus in the arbuscular mycorrhizal-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97: 163–182 [Google Scholar]
- Walker C, Sanders FE. (1986) Taxonomic concepts in the Endogonaceae: III. The separation of Scutellospora gen. nov. from Gigaspora Gerd. and Trappe. Mycotaxon 27: 169–182 [Google Scholar]
- Walker C, Schüssler A. (2004) Nomenclatural clarifications and new taxa in the Glomeromycota. Mycological Research 108: 979–982 [DOI] [PubMed] [Google Scholar]