Abstract
Background and Aims
Differential responses of closely related species to submergence can provide insight into the evolution and mechanisms of submergence tolerance. Several traits of two wetland species from habitats with contrasting flooding regimes, Rorippa amphibia and Rorippa sylvestris, as well as F1 hybrid Rorippa × anceps were analysed to unravel mechanisms underlying submergence tolerance.
Methods
In the first submergence experiment (lasting 20 d) we analysed biomass, stem elongation and carbohydrate content. In the second submergence experiment (lasting 3 months) we analysed survival and the effect of re-establishment of air contact on biomass and carbohydrate content. In a separate experiment we analysed expression of two carbohydrate catabolism genes, ADH1 and SUS1, upon re-establishment of air contact following submergence.
Key Results
All plants had low mortality even after 3 months of submergence. Rorippa sylvestris was characterized by 100 % survival and higher carbohydrate levels coupled with lower ADH1 gene expression as well as reduced growth compared with R. amphibia. Rorippa amphibia and the hybrid elongated their stems but this did not pay-off in higher survival when plants remained submerged. Only R. amphibia and the hybrid benefited in terms of increased biomass and carbohydrate accumulation upon re-establishing air contact.
Conclusions
Results demonstrate contrasting ‘escape’ and ‘quiescence’ strategies between Rorippa species. Being a close relative of arabidopsis, Rorippa is an excellent model for future studies on the molecular mechanism(s) controlling these strategies.
Keywords: Rorippa, submergence tolerance, escape and quiescence strategies, flooding, carbohydrate reserves, survival
INTRODUCTION
The frequency of major floods has increased significantly in the last century and this trend is predicted to continue due to climate change (Milly et al., 2002). Although modern terrestrial plants evolved from aquatic ancestors, very few angiosperm species can cope with the severe effects of flooding (Voesenek et al., 2006), which can lead to crop failure and demise of natural plant populations (Silvertown et al., 1999; Normile, 2008). The combined effects of limited underwater gas diffusion (Jackson, 1985) and low light levels in turbid waters (Vervuren et al., 2003) lead to mortality typically within just a few days of submergence. Thus, submergence acts as a strong selection force controlling the distribution of plant species in flooded areas (Blom, 1999; Van Eck et al., 2004; Mommer et al., 2006a).
Plants that inhabit frequently flooded areas have evolved traits to overcome the lethal effects of submergence. Two strategies have been proposed to cope with flooding, the low-oxygen escape strategy and the low-oxygen quiescence strategy. In the low-oxygen escape strategy, the plant grows and/or elongates its shoot in an attempt to reach the surface and restore air contact (Bailey-Serres and Voesenek, 2008). Plants adopting this strategy often have more internal aerenchyma tissue for efficient gas transport, especially to the below-ground parts where the effects of low-oxygen stress are most severe (Sauter, 2000; Voesenek et al., 2003). This strategy is beneficial only in shallow and prolonged floods, when plants are able to reach the surface before the stress becomes lethal. Since low levels of oxygen upon submergence can limit aerobic respiration, plants switch to anaerobic lactic acid and ethanol fermentation as a source of NAD+ and ATP, and carbohydrate catabolism genes such as ADH, PDC, SUS are up-regulated (Bailey-Serres and Voesenek, 2008). Compared with aerobic respiration, fermentation is far less efficient in terms of carbohydrate consumption. Plants can temporarily withstand submerged conditions; however, if air contact cannot be established, high carbohydrate consumption to accommodate stem elongation and cell division will lead to an energy deficit, severe tissue damage and mortality (Pierik et al., 2009; Chen et al., 2011). In contrast to the escape strategy, the low-oxygen quiescence strategy is defined by reduced levels of growth, conservation of energy reserves and a delay of the energy crisis (Setter and Laureles, 1996; Sauter, 2000; Bailey-Serres and Voesenek, 2008). This strategy is particularly beneficial when floods are deep but transient. In these conditions, rapid growth by the plant would be futile and likely to lead to stem breakage when water levels drop (Chen et al., 2011).
The escape and quiescent strategies have been well described in varieties of rice cultivated in different habitats. Deep-water rice can grow up to 4 m when flooded (Kende et al., 1998) in contrast to lowland rice that almost completely ceases its growth upon flooding and survives longer by conserving carbohydrates (Ram et al., 2002). Molecular studies have shown that ethylene response factor genes SNORKEL and SUB1A control escape and quiescence strategies, respectively (Fukao et al., 2006; Xu, 2006; Hattori, 2007, 2008; Hattori et al., 2009). Rice and arabidopsis studies have increased our fundamental molecular knowledge about submergence tolerance; however, neither species exhibits an extreme flooding tolerance phenotype. Submergence-tolerant lowland cultivars of rice and Arabidopsis thaliana accessions can survive only up to 10–14 d of submergence (Jackson and Ram, 2003; Vashisht et al., 2011). Introgression of the SUB1 locus that improves survival by only a few days to rice cultivars has already increased yield significantly after flooding (Singh et al., 2009), so identifying that the molecular basis of extreme tolerance would be of great interest for agriculture.
Rice varieties displaying these strategies are artificially selected genotypes and there is little evidence that these cost–benefit patterns also exist as a result of natural selection for different flooding regimes. Rumex species show variation in ethylene-induced petiole elongation depending on the type of floods they encounter in their natural habitats (Voesenek et al., 1996). One of these species, Rumex palustris elongates its leaves under submerged conditions, and there also exists variation in elongation ability among different populations (Chen et al., 2009). Petiole elongation and reaching water surface have benefits in biomass recovery (Pierik et al., 2009; Chen et al., 2011) but no evidence has been reported for effects on survival.
Studies on tolerant Rumex species have led to the discovery of fundamental knowledge about hormonal regulation pathways of elongation and consequences of submergence (Voesenek et al., 1991, 2003; Benschop et al., 2005; Vreeburg et al., 2005; Mommer et al., 2006a; Chen et al., 2009, 2011); however, the lack of molecular resources makes it hard to reveal the genetic mechanisms behind these responses in the detail possible for model organisms. Applicability of molecular tools developed for arabidopsis to its wild relatives (Mitchell-Olds, 2001; Schranz et al., 2007) provides greater possibilities for unravelling the genetic basis of extreme submergence tolerance. In this study, we used three wild relatives of arabidopsis belonging to the same lineage in the Brassicaceae (Al-Shehbaz et al., 2006), Rorippa amphibia, Rorippa sylvestris and their hybrid as models to study mechanisms of extreme submergence tolerance. The cytotypes studied were tetraploids with 32 chromosomes, most likely with an autoploid origin (Stift et al., 2010). These species are clonal wetland perennials, inhabiting major river plains in Europe. Rorippa amphibia usually occurs at stable water-tables, with waterlogged below-ground plant parts, whereas R. sylvestris occupies river beaches and habitats that undergo periodic deep floods and dry-outs (Jonsell, 1968; Blom, 1999). Natural interspecific hybrids can be found in intermediate habitats along major rivers that experience periodic flooding, such as the Danube and Elbe. A previous study with these species found differential responses to waterlogging and submergence (Stift et al., 2008). Stift et al. (2008) showed that R. amphibia was better able to cope with waterlogging. When completely submerged for 2 weeks, above-ground biomass of R. amphibia increased at the expense of decreased below-ground biomass. The above-ground and below-ground growth trade-off was not observed in R. sylvestris. This study suggested that these two species evolved different responses due to the different flooding regimes they encounter in their natural habitats. But are these differences shaped by natural selection? And, if so, what are the components that enhance or decrease submergence survival? To answer these questions we investigated the submergence responses and strategies of R. amphibia, R. sylvestris and their artificial F1 hybrid, by measuring changes in growth, biomass, carbohydrate metabolism, regulation of genes related to carbohydrate catabolism (ADH1 and SUS1) and their effects on survival. To understand the genotypic influence on the trait phenotypes, we tested to see if the hybrid behaved like either of the parents and if so how these were reflected in survival.
MATERIALS AND METHODS
Plant material
The genotypes of Rorippa amphibia, R. sylvestris and their artificial hybrid R. × anceps used in this study were previously described in Stift et al. (2008). Rorippa amphibia and R. sylvestris rhizomes were collected from the River IJssel, Doesburg, The Netherlands (52 °01′25″N, 06 °08′42″E) and the River Rhine, Millingerwaard, The Netherlands (51 °52′02″ N, 05 °59′18″E), respectively. All plants used were tetraploids (4n = 32). The F1 hybrids were derived by hand-pollination using the R. amphibia plant as the pollen donor and the R. sylvestris as the maternal plant. Five random seeds were selected from the crosses and were germinated on sterile filter paper with 2 mL of 3 m gibberellic acid solution. Seedlings were transferred to soil and grown for 3 months. Intermediate morphology of the progeny confirms that the crosses were successful and hybrids were created (see Supplementary Data Fig. S5). One random hybrid genotype was selected and together with the parents propagated by rhizome cuttings for 2 years. Replicate plants used in all experiments were genetically identical clones propagated from rhizomes. The rhizomes were collected and surface sterilized by using 10 % (v/v) bleach solution for 8 min and washed three times with deionized water. The rhizomes were later cut in 2- to 3-cm fragments and placed on 0·8 % agar (Hispanagar, Burgos, Spain) and 0·5X MS (Duchefa, Haarlem, The Netherlands) media. Rhizomes were initially grown in a growth cabinet (Sanyo MLR-350; Sanyo, Etten-Leur, The Netherlands) for 10 d with 16 h light at 20 °C and 8 h dark at 16 °C. Subsequently, individual plantlets were transferred onto 55-mm-diameter mesh pots with sterile sand (0·5- to 1·0-mm grain size; Filcom BV, Papendrecht, The Netherlands) with nutrient solution (0·1 g l−1; Peters Professional 20 : 10 : 20 General purpose; Scotts Europe BV, Heerlen, The Netherlands). The plants were grown in a greenhouse under natural light supplemented with 600-W SON-T lamps (Philips, Eindhoven, The Netherlands) when necessary. The temperature of the greenhouse was 20 °C (±2 °C) with a 16-h photoperiod. After 2 weeks plants were transferred to 3-L pots containing 1·5 g (per pot) controlled slow-release fertilizer (Osmocote Plus 15 + 11 + 13 + 2MgO + trace elements; Peters Professional, Scotts Europe BV).
First submergence experiment
This experiment was conducted in May–June 2008. After 50 d of pre-growth, the stem lengths of 36 plants per genotype used in the experiment were measured. Plants were placed in four outdoor cement basins on 36 randomly assigned positions in each basin (length × width × depth = 400 cm × 100 cm × 100 cm). The plants were either submerged completely or left in empty basins as air controls. All the basins were covered with shade cloth to prevent plants from emerging and to mimic deep flooding light conditions. Light intensity was measured with a light data logger (LI-1400; Licor, Lincoln, NE, USA) and light under the shade cloth was found to be 65 % of the normal light intensity. At the start of the experiment and 7, 14, 20 d after the treatment started, stem lengths were measured and above-ground and below-ground tissues of six plants from each genotype and treatment were washed and sampled separately. Dry biomass was measured after freeze-drying and a subsample was used for carbohydrate analysis.
Second submergence experiment
In the summer of 2009 a similar but longer-term submergence experiment was performed. After 51 d of pre-growth 117 plants per genotype were randomly placed in six cement basins. At the start of the treatment, above-ground and below-ground tissues of nine plants of each genotype were washed and sampled separately for carbohydrate analysis. All the plants were submerged completely and shade cloth was used to cover the basins. After 37 d of complete submergence, a further nine plants per genotype were sampled (for carbohydrate analysis) and the water level in half of the basins (three basins) was lowered to half of the original height to let all genotypes gain contact with the atmosphere (semi-submergence treatment). Nine completely submerged and nine semi-submerged plants were harvested 21 and 42 d after the water level was lowered for dry weight and carbohydrate analyses. Forty-two days after reaching the surface, some plants were lethally damaged; nevertheless we were able to sample both above-ground and below-ground tissues. For all samples dry biomass was measured after freeze-drying and a sub-sample was used in carbohydrate analysis.
For survival assays, separate sets of 12 plants from each genotype were taken out of the water after 37, 58 and 79 d of complete submergence, as well as plants submerged for 37 d followed by 21 and 42 d of semi-submergence. Survival was scored both immediately and after a recovery period of 15 d, based on absence or presence of green parts above ground. In the survival data only immediate survival was included for R. amphibia and the hybrid since the stems were very weak and unable to support the large healthy shoot tissues when the water was removed.
Gene expression experiment
After 30 d of pre-growth, 48 plants from each genotype were randomly assigned to 16-L plastic buckets either completely filled with rainwater 1 d before the start of the experiment (submergence treatment) or with 1 cm of water for air controls. This experiment was conducted in a greenhouse with the same conditions as the growth period. After 3 d of complete submergence, the water level was reduced in half of the buckets from the submergence treatment so that tips of the leaves were emerging. Four plants were harvested for each genotype and treatment 2, 26, 74 h after the water level was lowered. For air controls and submergence treatments the plants were quickly rinsed in water; roots and shoots were sampled separately. For semi-submerged samples roots, leaves above water level and shoots under water were sampled separately. All the samples were quickly frozen in liquid N2 for RNA isolations and expression analysis.
Carbohydrate analysis
A sub-sample of 10–150 mg of ground tissue (sampled in the first and second experiments for carbohydrate analysis) was suspended in 1·0 mL 70 % MeOH in water (v/v), vortexed and boiled for 5 min. After placing the tubes in an ultrasonic bath for 15 min, samples were centrifuged (10 min at 16 000 g) and the supernatants were transferred to new tubes. Pellets were extracted once more, excluding the boiling step. Supernatants of each sample were combined and 70 % MeOH was used to bring the final volume to 2 mL. For HPLC quantification, 10 µL of extract was diluted in 990 µL of MilliQ water and measurements and data analysis were performed as described previously (Van Leur et al., 2008). For starch measurements, the pellets were mixed with 1 mL of water and incubated at 65 °C for 30 min. The supernatants were transferred to new tubes after centrifugation at 23 000 g for 5 min and 1 mL water was added to the pellets once more. The supernatants were combined with the previous fractions. The pellets were then boiled in a water bath for 10 min. After cooling to room temperature, 500 µL of 0·2 m Na acetate (pH 5·5), 7 units amyloglucosidase and 0·7 units α-amylase were added to the pellets and incubated at 37 °C for 4 h. After centrifugation at 27 000 g for 5 min the supernatants were transferred to new tubes for starch analysis. The hexose concentration was measured by a modified version of the anthrone method by using fixed glucose standards (Smith and Zeeman, 2006).
Gene cloning and expression analysis
DNA was isolated with a DNeasy Plant mini kit (Qiagen, Leusden, The Netherlands) from R. amphibia and R. sylvestris. Primers for ADH1 and SUS1 were designed by Primer3 software (http://frodo.wi.mit.edu/primer3/primer3_code.html) based on arabidopsis sequences obtained from TAIR. Amplified fragments with these primers from gDNA of R. amphibia and R. sylvestris were cloned with standard protocols (Sambrook and Russell, 2001). Based on sequences from at least ten independent clones for each gene per species, qPCR primers were designed for conserved regions among species. Primers were blasted to search for similar genes in A. thaliana to confirm specificity. Primers for 18S rRNA, used as a reference gene for qRT-PCR, were designed based solely on arabidopsis sequences. The primers used in qRT-PCR were (5′-3′)
18S rRNA-forward AAACGGCTACCACATCCAAG
18S rRNA-reverse ACTCGAAAGAGCCCGGTATT
ADH1-forward GGACTTGGTGCTGTTGGTTTAG
ADH1-reverse CTGGTTTGTCATGCTCTCTCG
SUS1-forward GGAGAGTTTGCTTCCATTGC
SUS1-reverse TCCGCTTTCCTCAAGATGTG
Primer specificity was assessed by melting and dilution curve analysis. Each primer set amplified only one product (Supplementary Data Fig. S4). RNA isolations were done with a modified version of the hot phenol method (Slater, 1984). Genomic DNA was digested with DNase (DNA-free; Ambion, Nieuwerkerk aan de lJssel, The Netherlands) and cDNA was synthesized with 500 ng RNA, 50 ng random hexamers (Invitrogen, Bleiswijk, The Netherlands) and 100 U SuperScript III reverse transcriptase (Invitrogen, Bleiswijk, The Netherlands) according to manufacturer's instructions. Quantitative PCR reaction mixtures included 2X SYBR green (Platinum SYxBR green Supermix gPCR UDG; Invitrogen, Bleiswijk, The Netherlands), 0·3 µL 10 µm of each primer, 0·04 µL 1/10 dilution 50× ROX reference dye and 10 ng cDNA, except 0·001 ng cDNA was used for 18S rRNA in a total volume of 20 µL. The reaction was performed with a real-time PCR system (Applied Biosystems, CA, USA) and relative expression levels were calculated using the ΔΔCt method (Livak and Schmittgen, 2001) and corrected for 18S rRNA transcript levels. The data were log2 transformed and then used in the contrast analysis.
Porosity measurements
Porosity was measured as volume air space as a percentage of total tissue volume according to Raskin (1983) and using calculations as adapted by Thomson et al. (1990).
Statistical analysis
All analyses were performed with SPSS 16·0 for Mac (SPSS Incorporated, Chicago, IL, USA). We performed ANOVA analysis to test treatment, time-point and species effects for both submergence experiments (Supplementary Data Tables S1 and S2). Three contrast analyses were performed for both first and second experiments. The difference between starting values (day 0) and submerged values (20 d for first and 37 d for second experiments) were contrasted with an ANOVA test among R. amphibia and R. sylvestris, R. amphibia and the hybrid and lastly R. sylvestris and the hybrid. The trait was considered additive when there was a difference in the parental lines and the hybrid was intermediate and differed from both (A > H > S or S > H > A). When the hybrid was different from one parent only, the trait was considered dominant. If the hybrid exceeded any of the parental lines, trait was evaluated as over-dominant. For the gene expression experiment, three contrasts were performed to test if the pattern of gene expression was different among species. In the contrast analysis, all time-points were included and values for one species at a time point was contrasted against the same time point for other genotypes. The contrasts for following comparisons were done: air controls vs. submerged samples, air controls vs. semi-submerged samples and submerged vs. semi-submerged samples.
RESULTS
Rorippa sylvestris displayed a quiescence strategy, whereas Rorippa amphibia and the hybrid displayed an escape strategy upon submergence
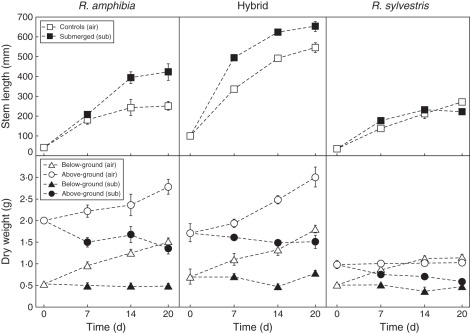
All genotypes exhibited at least some stem elongation under submerged conditions (Fig. 1). Compared with air controls, submerged R. amphibia showed increased stem elongation, whereas no difference was found between treatments in R. sylvestris. The hybrid had the tallest stems under both normal and submerged conditions, reaching more than twice the height of both of the parental lines. However, the relative treatment effect was not as strong as it was in R. amphibia: submerged R. amphibia plants showed 82 % greater stem elongation (length submerged/length in air) compared with 24 % in the hybrid after 20 d of submergence.
Fig. 1.
Stem elongation of Rorippa amphibia, Rorippa sylvestris and their hybrid (top), and above-ground and below-ground dry weight (bottom) of control and submerged plants after 7, 14 and 20 d of treatment (n = 6; bars indicate s.e.).
Above-ground biomass was reduced in all species after submergence (Fig. 1). The reduction of biomass in R. amphibia and the hybrid was comparable to that in R. sylvestris (Table 1). Growth of below-ground tissue in all the genotypes ceased upon submergence, and there were no significant differences in below-ground biomass after 20 d of submergence compared with starting values (Supplementary Data Table S1).
Table 1.
Summary of pair-wise comparisons of the response to 20 d of submergence (first experiment) of the parental lines and their hybrid, using ANOVA contrasts; for instance, the increase in stem length from day 0 till day 20 (of submergence) differs among all three groups (cf. the slopes in Fig. 1, stem length)
|
F-value contrast |
||||
|---|---|---|---|---|
| Response variable | R. amphibia vs. R. sylvestris | R. amphibia vs. hybrid | R. sylvestris vs. hybrid | Conclusion |
| Stem length (mm) | 21·33*** | 16·12*** | 74·52*** | H > A > S A + over-dominance |
| BG d. wt (g) | 0·03 | 0·65 | 0·39 | H = A = S Inconclusive |
| AG d. wt (g) | 0·91 | 2·91 | 0·56 | H = A = S Inconclusive |
| AG glucose (mg g−1 d. wt) | 0·54 | 0·37 | 1·72 | H = A = S Inconclusive |
| AG fructose (mg g−1 d. wt) | 17·58*** | 5·68* | 3·27 | H = S < A S dominance |
| AG sucrose (mg g−1 d. wt) | 11·00*** | 4·32* | 1·53 | H = S > A S dominance |
| AG starch (mg g−1 d. wt) | 15·81*** | 25·92*** | 1·24 | H = S > A S dominance |
| BG glucose (mg g−1 d. wt) | 1·57 | 12·83*** | 23·38*** | H > S = A Over-dominance |
| BG fructose (mg g−1 d. wt) | 0·04 | 20·15*** | 22·02*** | H > S = A Over-dominance |
| BG sucrose (mg g−1 d. wt) | 0·04 | 6·56* | 5·61* | H > A = S Over-dominance |
| BG starch (mg g−1 d. wt) | 3·87 | 3·65 | 0·00 | H = A = S Inconclusive |
| BG total carbohydrates (mg g−1 d. wt) | 3·18 | 8·26* | 1·19 | H ≥ S = A Over-dominance |
| AG total carbohydrates (mg g−1 d. wt) | 9·19* | 26·76*** | 3·41 | H = S > A S dominance |
BG, below-ground; AG, above-ground; d. wt, dry weight; A, Rorippa amphibia, S, Rorippa sylvestris, H, hybrid.
* P < 0·05; ** P < 0·005; *** P < 0·0005 (highlighted in bold).
Rorippa amphibia had a higher petiole porosity compared with R. sylvestris, indicating more aerenchyma tissue, whereas the hybrid showed intermediate levels (Fig. 2 and Supplementary Data Tables S3 and S4). More aerenchyma tissue coupled with stem elongation enables greater internal gas diffusion rates as soon as plants re-establish contact with the atmosphere. The growth and morphology upon submergence thus suggest that R. amphibia and the hybrid display escape strategies, while R. sylvestris has a quiescence strategy.
Fig. 2.
Aerenchyma in petioles of 30-d-old control non-submerged plants of (A) Rorippa amphibia, (B) Rorippa sylvestris and (C) their hybrid, and (D) porosity content of 21-d-old petioles. All three genotypes differ in their porosity levels significantly (ANOVA, LSD tests; see Supplementary Data Tables S3 and S4) (n = 4 or 5; bars indicate s.e.).
Above-ground carbohydrates decreased more in R. amphibia upon submergence
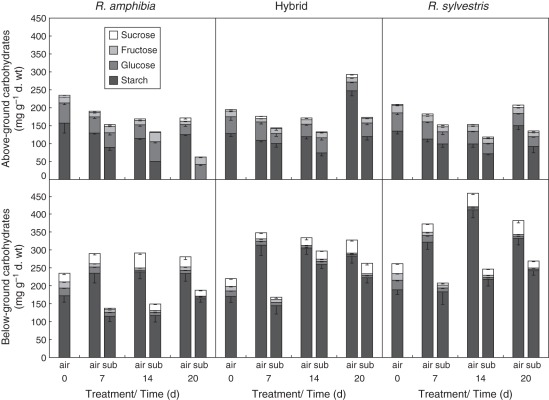
Total above-ground carbohydrate content decreased in all the genotypes upon submergence relative to air controls (Fig. 3 and Supplementary Data Table S2), probably as a result of energy deficit caused by oxygen deficiency and a shift from aerobic respiration to anaerobic metabolism. This reduction was strongest in R. amphibia; the above-ground starch concentration was reduced to zero after 20 d of submergence. Although there also was a decrease in carbohydrates in R. sylvestris compared with air controls, total carbohydrate content was maintained close to starting levels. This also applied to the hybrid (Table 1). Submerged R. amphibia had higher glucose and fructose concentrations compared with air controls, while sucrose concentration was reduced (Fig. 3 and Supplementary Data Fig. S1A). This trend was not found in R. sylvestris.
Fig. 3.
Soluble carbohydrates and starch content of (top) above-ground and (bottom) below-ground tissues of Rorippa amphibia, Rorippa sylvestris and their hybrid in air controls and submerged plants after 7, 14, 20 d of treatment (n = 6; bars indicate s.e.).
All genotypes showed a rapid reduction in total below-ground carbohydrates in the first week of submergence
In the first week of submergence there was an initial reduction in below-ground carbohydrates in all the genotypes, with R. amphibia showing the greatest reduction (Fig. 3). Carbohydrate levels subsequently increased again in all genotypes later on. The hybrid showed the smallest reduction in soluble carbohydrates and displayed a faster recovery after 14 d of submergence when compared with either of the parental lines (Table 1). At the end of the treatment, total below-ground carbohydrates were therefore highest in the hybrid, followed by R. sylvestris.
Carbohydrate catabolism genes are differentially regulated
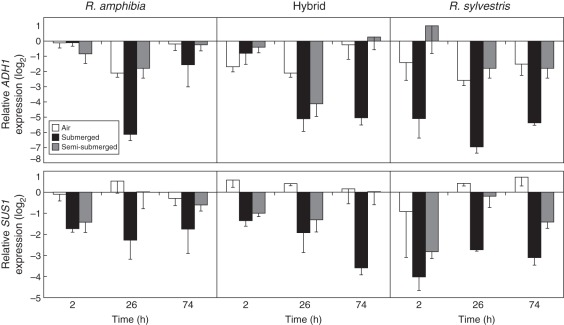
Partial sequences of ADH1 and SUS1 Rorippa homologues (genebank nos JQ582800, JQ582801, JQ582802, JQ582803) constituted 377 and 297 amino acids, respectively. Homology between A. thaliana ADH1 and the most common allele of Rorippa was 94 %, and 99 % for SUS1 (Supplementary Data Figs S6 and S7). After 3 and 6 d of complete submergence, R. amphibia displayed high ADH1 transcript levels similar to air controls (Fig. 4). However, R. sylvestris showed a stronger down-regulation, in contrast to hybrids, which showed a similar pattern to that of R. amphibia (Table 2). Three days after plants were allowed to establish air contact (74 h), ADH1 levels were similar to air controls in R. amphibia and the hybrid; however, this effect was less apparent in R. sylvestris. There was no differential regulation for SUS1 among species (Fig. 4), as all genotypes showed a down-regulation in submerged conditions and an up-regulation upon reaching water surface. Transcript levels of SUS1 and ADH1 showed a good correlation (R2 = 0·4385) supporting similar regulation mechanisms (Supplementary Data Fig. S3). The effects of treatments and genotypes were not as pronounced in above-ground tissues (data not shown).
Fig. 4.
(Top) ADH1 and (bottom) SUS1 expression in Rorippa amphibia, Rorippa sylvestris and the hybrid roots following 2, 26, 74 h of complete and semi-submergence after 3 d of complete submergence (n = 4; bars indicate s.e.). Rorippa amphibia air control at 2 h was selected as the reference (log2 = 0) for both genes.
Table 2.
Summary of pair-wise differences for contrast analysis between parental lines and the hybrid for ADH1 expression levels in the below-ground tissues (expression experiment)
|
F-value contrast |
|||
|---|---|---|---|
| Relative ADH1 expression | R. amphibia vs. R. sylvestris | R. amphibia vs. hybrid | R. sylvestris vs. hybrid |
| Air vs. submerged | 2·68† | 2·05 | 2·93† |
| Submerged vs. semi-submerged | 0·89 | 4·37† | 1·93 |
| Air vs. semi-submerged | 0·49 | 1·44 | 2·69† |
† P < 0·055 (highlighted in bold).
Escapers benefited from elongation only if they reached the water surface
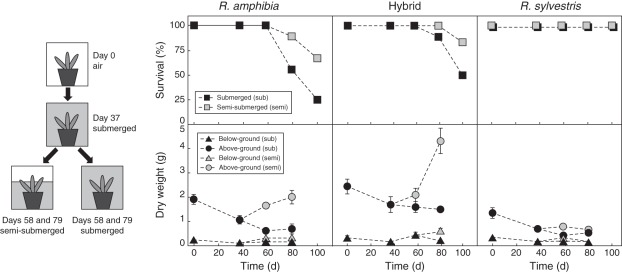
All three species had extreme tolerance to submergence, as evident from their survival in the long-term experiment. Rorippa sylvestris showed 100 % survival after up to 100 d of complete and semi-submergence (Fig. 5). No mortality was observed in either R. amphibia or the hybrid after 58 d, and only after 79 d the effects of complete submergence without air contact became evident, with R. amphibia being the least tolerant of the three genotypes. The restoration of shoot air contact after the period of complete submergence led to a lower mortality in both escapers. Rorippa amphibia still had high levels of mortality after 42 d of semi-submergence following the 37 d of complete submergence. The hybrid was more tolerant than R. amphibia; mortality was lower both in complete and semi-submergence treatments.
Fig. 5.
Schematic view of experimental timeline (left) . (Top) Survival and (bottom) above-ground and below-ground dry weight of plants at start and after 37 d of complete submergence followed by controlled air contact for 21 and 42 d for Rorippa amphibia, Rorippa sylvestris and their hybrid (n = 12 for survival, n = 9 for dry weight; bars indicate s.e.).
Only escapers benefitted in terms of biomass accumulation when reaching the water surface
There was a significant reduction in mainly above-ground biomass in all genotypes after 37 d of complete submergence (Fig. 5). After restoring air contact (semi-submergence), biomass increased significantly in the escaper species. Reaching the surface mostly led to above-ground biomass accumulation, although the effect was less apparent in below-ground tissues. Remarkably, reaching the surface had almost no effect on quiescent R. sylvestris in terms of biomass recovery.
No increase in above-ground carbohydrate content occurred upon reaching the water surface
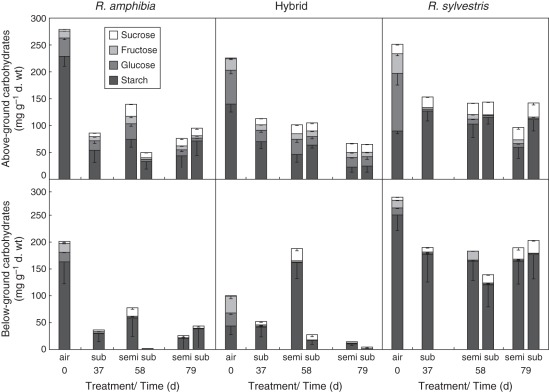
After 37 d of complete submergence, total above-ground carbohydrates were reduced in all genotypes and again more so in R. amphibia (Fig. 6 and Table 3) similarly to the first experiment (Fig. 3). Above-ground glucose and fructose content (Supplementary Data Fig. S2B) was reduced in all genotypes and most dramatically in R. sylvestris after 79 d of complete submergence. The hybrid showed a similar trend to R. sylvestris in glucose and fructose reduction (Table 3). Sucrose levels significantly increased in above-ground tissues of all genotypes, but more so in R. sylvestris (Table 3). The reduction in starch levels was strongest in R. amphibia. Following 37 d of complete submergence, an increase in carbohydrates was observed only in R. amphibia after 21 d of air contact. However, after 42 d of air contact, there was no clear benefit in terms of carbohydrate levels from reaching the surface in any of the species. Nevertheless, the biomass increase upon air contact establishment led to a higher total amount of carbohydrates in the escapers; although the carbohydrate concentrations did not change (as shown in Fig. 6), the total amount within the plant increased as a result of increased biomass. Sucrose levels increased further during the first 21 d of air contact in R. amphibia, whereas R. sylvestris and the hybrid showed a continuous increase in sucrose levels regardless of being submerged or semi-submerged (Supplementary Data Fig. S2A, B). We also observed increased trehalose levels in roots of all genotypes after 79 d of complete submergence (Supplementary Data Fig. S2B).
Fig. 6.
Soluble carbohydrate and starch content of (top) above-ground and (bottom) below-ground tissues at start and after 37 d of complete submergence followed by controlled air contact for 21 and 42 d for Rorippa amphibia, Rorippa sylvestris and their hybrid (n = 9; bars indicate s.e.).
Table 3.
Summary of contrast analysis between parental genotypes and the hybrid for the change from starting values (day 0) to 37 d of submergence (second experiment); for instance, the decrease in above-ground d. wt from day 0 till day 37 (of submergence) does not differ among all three groups (cf. the slopes in Fig. 5, dry weight)
|
F-value contrast |
||||
|---|---|---|---|---|
| Response variable | R. amphibia vs. R. sylvestris | R. amphibia vs. hybrid | R. sylvestris vs. hybrid | Conclusion |
| BG d. wt (g) | 0·49 | 0·69 | 0·02 | H = S = A |
| AG d. wt (g) | 0·26 | 0·09 | 0·04 | H = S = A |
| AG glucose (mg g−1 d. wt) | 4·33* | 10·91** | 1·50 | H = S < A S dominance |
| AG fructose (mg g−1 d. wt) | 16·14*** | 11·59** | 0·38 | H = S < A S dominance |
| AG sucrose (mg g−1 d. wt) | 46·95*** | 9·15** | 14·64*** | S > H > A Additivity |
| AG starch (mg g−1 d. wt) | 10·58** | 10·71** | 0·00 | H = S > A S dominance |
| BG glucose (mg g−1 d. wt) | 1·06 | 1·87 | 5·75* | H ≥ A = S Over-dominance |
| BG fructose (mg g−1 d. wt) | 0·35 | 6·86* | 10·33** | H > S = A Over-dominance |
| BG sucrose (mg g−1 d. wt) | 2·27 | 1·05 | 0·23 | H = S = A Inconclusive |
| BG starch (mg g−1 d. wt) | 8·39** | 7·16* | 0·10 | H = S > A S dominance |
| BG total carbohydrates (mg g−1 d. wt) | 8·47** | 4·47* | 0·82 | H = S > A S dominance |
| AG total carbohydrates (mg g−1 d. wt) | 7·08* | 5·24* | 0·14 | H = S > A S dominance |
BG, below-ground; AG, above-ground; d. wt, dry weight; A, Rorippa amphibia, S, Rorippa sylvestris, H, hybrid.
* P < 0·05, ** P < 0·005, *** P < 0·0005 (highlighted in bold).
Quiescent R. sylvestris showed little reduction of below-ground carbohydrate levels after prolonged submergence
Carbohydrate levels were significantly reduced in below-ground tissues of all the genotypes after 37 d of complete submergence, but this was particularly striking in R. amphibia (Fig. 6 and Table 3). At longer periods of complete submergence, further reductions in below-ground carbohydrate levels occurred in both R. amphibia and the hybrid. Rorippa sylvestris maintained more of its below-ground carbohydrates and showed <30 % reduction after 100 d of submergence, compared with 78 % and 95 % reductions in R. amphibia and the hybrid, respectively. After 21 d of air contact, carbohydrates had increased in all the genotypes, but more significantly in the escaper genotypes. However, the effects were no longer evident after 42 d of air contact.
Similar to above-ground tissue, below-ground sucrose levels increased in all species upon submergence. After 37 d, R. sylvestris roots had more sucrose than any of the other genotypes (Supplementary Data Fig. S2B). Sucrose levels were higher after 21 d of air contact in the escapers, but after 42 d of air contact, levels were comparable to submerged plants. Rorippa sylvestris maintained higher underground starch levels regardless of being fully or semi-submerged.
DISCUSSION
Flooding can shape plant species distributions along a submergence gradient and can act as a strong selective pressure for key adaptations, potentially leading to speciation (Keddy, 1984; Silvertown et al., 1999; Vervuren et al., 2003; Van Eck et al., 2004; Lenssen and De Kroon, 2005; Voesenek et al., 2006). We have shown that two wetland species Rorippa amphibia and Rorippa sylvestris from habitats with different flooding regimes, display different tolerances and survival strategies for submergence stress: R. amphibia has adopted the escape strategy and R. sylvestris the quiescence strategy.
Yellowcress species have contrasting strategies to cope with flooding
When submerged, R. amphibia displays an escape strategy defined by prolonged stem elongation in an attempt to reach the surface and supply the submerged tissues with oxygen necessary for respiration. Supported by aerenchyma tissue, this strategy could be very beneficial when floods are long lasting and relatively shallow, allowing plants to restore air contact and thus internal aeration (Bailey-Serres and Voesenek, 2008). In contrast, R. sylvestris displays a quiescence strategy by minimizing its growth and conserving carbohydrate reserves. Since R. sylvestris occupies habitats with deeper and transient floods (Jonsell, 1968; Blom, 1999), the quiescence strategy can increase the fitness of the plant by avoiding consumption of carbohydrates in growth processes. The high levels of carbohydrates we measured in this species even after 79 d of complete submergence suggest that underwater photosynthesis may also play an important role in survival of R. sylvestris (Mommer and Visser, 2005; Mommer et al., 2006b; Stift et al., 2008). We observed increasing levels of sucrose in below-ground tissues of all the genotypes under submerged conditions but more in of R. sylvestris. Sucrose plays an important role in the onset of the quiescence strategy in rice (Kudahettige et al., 2011) and in submergence tolerance of Arabidopsis thaliana (Loreti et al., 2005) and might also be crucial for the extreme submergence tolerance in Rorippa. Increasing sucrose levels in shoots might be a result of efficient underwater photosynthesis, which would enhance tolerance.
After 5 weeks of complete submergence R. sylvestris had larger starch reserves than either of the other genotypes, both above and below ground. A previous study of R. amphibia and R. sylvestris roots using arabidopsis GeneChip microarrays showed that genes related to carbohydrate catabolism (SUS1, ADH1, PDC1) are up-regulated in all genotypes upon 24 h of submergence, but to a greater extent in R. amphibia (A. Boonman et al, unpubl. res.). In addition to this initial up-regulation, we showed ADH1 and SUS1 are down-regulated at later stages of complete submergence in R. sylvestris. It has also been shown that in A. thaliana, ADH and PDC1 are initially up-regulated under anoxia but levels decline at later stages (Loreti et al., 2005). However, in R. amphibia, ADH1 and SUS1 transcript levels were high even after 6 d of complete submergence in contrast to the low levels in R. sylvestris. This might explain the lower consumption of carbohydrates and a higher survival in R. sylvestris. The quiescence strategy controlled by SUB1A-1 in rice is also defined by lower growth rates and conservation of carbohydrates in spite of induction of fermentation genes (Fukao et al., 2006). The high correlation in transcript levels of ADH1 and SUS1 in different treatments and genotypes supports the suggestion that these pathways are co-regulated in Rorippa.
Stem elongation can be an efficient process to reach the surface. Nevertheless, growing tissues need energy, which would lead to a faster depletion of carbohydrates by anaerobic metabolism under low oxygen (Bailey-Serres and Voesenek, 2008, 2010). Complete depletion of above-ground starch in R. amphibia after 20 d of submergence and higher ADH1 and SUS1 transcript levels support the fact that elongation demands high levels of energy (Groeneveld and Voesenek, 2003). Glucose and fructose levels were also higher in R. amphibia, possibly because of the breakdown of starch reserves for glycolysis and anaerobic metabolism to supply ATP for growth (Perata and Alpi, 1993; Guglielminetti et al., 1995; Perata et al., 1996).
In all genotypes, a rapid reduction of carbohydrates was observed within the first week of submergence, followed by increasing or stable levels in the later weeks. Consumed carbohydrates might be supplied to organs that undergo acclimations for submergence which can include newly formed leaves with more aerenchyma tissue and/or higher underwater photosynthesis ability and tissues more resistant to reactive oxygen species (Voesenek et al., 2006). The increase in carbohydrates after the second week also indicates that the plants acclimated to the submergence stress. As a result, plants mostly had healthy and green leaves even after 3 weeks of complete submergence, although there were significant effects of submergence on biomass and carbohydrate content in all the genotypes.
Although starch was completely depleted in 20 d, the carbohydrate levels recovered at a later stage (37 d of complete submergence). This may be the result of most of the stem elongation taking place in the first 2 weeks of submergence. After stem growth ceased, the plants might have accumulated more carbohydrates by increased underwater photosynthesis of the stem and its small leaves (Raskin and Kende, 1984; Beckett et al., 1988). Additionally, observed increases in trehalose levels might also have been a factor increasing submergence tolerance since this carbohydrate appears to improve tolerance to several abiotic stresses (Chen and Murata, 2002; Garg et al., 2002).
Hybrids are mostly escapers, and are able to elongate a stem while conserving carbohydrates
In growth and morphology, the hybrid displayed an escape strategy, although the effect of the treatment on stem elongation was not as strong as in R. amphibia. Surprisingly, the carbohydrate levels were overall comparably high compared with those in R. sylvestris. The hybrid had a similar level of above-ground carbohydrate reduction to R. sylvestris in the first experiment (after 20 d of submergence) and similar patterns of both above- and below-ground carbohydrate concentrations in the long-term experiment (after 37 d of submergence). This explains the higher survival of the hybrid compared with R. amphibia, and could be due to the presence of the mechanisms of the R. sylvestris parent for conserving carbohydrates, or due to more efficient underwater photosynthesis. Potentially, this could also lead to higher fitness of hybrids than their parents under certain field conditions. Hybrids are often found in flood plains where both parents are found, occupying intermediate locations (Bleeker, 2004, 2007). The intermediate levels of the hybrid in various traits suggest that neither of the parental strategies is dominant over the other, in agreement with such a distribution pattern in the wild (Bleeker and Hurka, 2001).
Quiescent R. sylvestris lacks the recovery mechanisms of the escape strategy after reaching the water surface
Although R. amphibia inhabits sites that can be completely submerged, it has a lower survival rate compared with R. sylvestris since the latter shows no mortality even after >3 months of submergence. The depth of submergence determines the stem length necessary to reach the surface and hence the carbohydrate demands for this elongation. It has been shown that reaching the water surface favours fitness-related traits (Pierik et al., 2009; Chen et al., 2011) but the effect on survival has never been shown before. If plants are able to reach the surface, survival is improved significantly in R. amphibia and the hybrid. Some plants showed mortality even after air contact was established; possibly because of the existing damage caused by the long complete submergence period or the sudden switch to normoxia, and resulting oxidative damage. Upon establishing air contact, surviving plants of both R. amphibia and the hybrid grew extensively above the water level. This phenotype was almost completely absent in R. sylvestris after partial air contact establishment. This suggests that a quiescent period in this species extends to a semi-submerged state, and that vigorous growth is resumed only after a further drop in the water-table, and restoration of normoxic conditions below ground. The lower porosity in R. sylvestris might explain the absence of a recovery after air restoration. Since the stem above the water surface cannot function as an efficient snorkel when sufficient aerenchyma is absent, the parts under water might remain anoxic for a longer time. We also observed that as soon as R. sylvestris was de-submerged completely, clones were emerging from rhizomes, forming new healthy plants within weeks (M. Akman, pers. obs.), possibly enhanced by the higher carbohydrate levels in below-ground tissues.
Submergence tolerance strategies and clonal growth
The escape and quiescent strategies of the species are correlated with other aspects of clonal growth. Although both of the species are rhizome sprouters (Jonsell, 1968), clonal growth from rhizomes is more vigorous in R. sylvestris, possibly stimulated by the high levels of carbohydrates in below-ground tissues. This trend is also observed under submerged conditions and can increase plant survival by means of rhizome sprouts after a prolonged flood even if above-ground tissues completely die. It has been shown that adventitious bud formation after a heavy injury increases fitness in Rorippa palustris (Klimesová and Klimes, 2007; Klimesova et al., 2008). The higher abundance of rhizome-sprouting clonal species in wetlands (Sosnova et al., 2010) might be due to higher survival achieved by this strategy.
During semi-submergence, we observed that the growing above-ground parts of the escapers were forming adventitious roots in stems and leaves, and were becoming detached from the slender underwater stem. This was also observed in naturally flooded areas, where emergent R. amphibia plants can become detached from the underwater stems, float around and settle at a different location (Jonsell, 1968). This slender and deteriorating stem might explain the low recovery in biomass of the root system compared with the vigorous above-ground tissues, due to inefficient transport of resources to below-ground tissues.
In conclusion, R. amphibia, R. sylvestris and their hybrid all exhibit extreme submergence tolerance, which is achieved by different strategies selected by their natural habitats. Escaper R. amphibia invests in elongating its stem and consumes its carbohydrate reserves in order to reach the floodwater surface and, if this is not established, plants from this species die sooner than those of their quiescent relative R. sylvestris, which shows a higher survival by limiting growth and conserving resources. Their hybrid also displays an escape strategy but at the same time conserves its carbohydrates better than R. amphibia and thus has lower mortality. Being close relatives of the model plant arabidopsis, Rorippa species constitute a good model for studying the molecular basis of extreme submergence tolerance with their escape and quiescence strategies. Although the mechanisms of these responses are largely unknown, the opportunities of using information from arabidopsis will accelerate the research to unravel the genetics underlying these strategies. Many advantages such as ease of cloning genes by using available arabidopsis sequence data and applicability of gene expression assays designed for arabidopsis increases the potential of Rorippa flooding research.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We would like to thank Rob Bregman and Peter Kuperus for their support in every step of this study and Nicole van Dam and Ciska Raaijmakers for assisting us with soluble carbohydrate analyses. We would also like to thank Ludek Tikovsky, Thys Hendriks and Harold Lemereis for taking care of our plants in the greenhouse and Jan van Arkel for preparation of aerenchyma tissue pictures.
LITERATURE CITED
- Al-Shehbaz IA, Beilstein MA, Kellogg EA. Systematics and phylogeny of the Brassicaceae: an overview. Plant Systematics and Evolution. 2006;259:89–120. [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. Life in the balance: a signaling network controlling survival of flooding. Current Opinion in Plant Biology. 2010;13:489–494. doi: 10.1016/j.pbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Beckett PM, Armstrong W, Justin SHFW, Armstrong J. On the relative importance of convective and diffusive gas flows in plant aeration. New Phytologist. 1988;110:463–468. [Google Scholar]
- Benschop JJ, Jackson MB, Gühl K, et al. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. The Plant Journal. 2005;44:756–768. doi: 10.1111/j.1365-313X.2005.02563.x. [DOI] [PubMed] [Google Scholar]
- Bleeker W. Genetic variation and self-incompatibility within and outside a Rorippa hybrid zone (Brassicaceae) Plant Systematics and Evolution. 2004;246:35–44. [Google Scholar]
- Bleeker W. Interspecific hybridization in Rorippa (Brassicaceae): patterns and processes. Systematics and Biodiversity. 2007;5:311–319. [Google Scholar]
- Bleeker W, Hurka H. Introgressive hybridization in Rorippa (Brassicaceae): gene flow and its consequences in natural and anthropogenic habitats. Molecular Ecology. 2001;10:2013–2022. doi: 10.1046/j.1365-294x.2001.01341.x. [DOI] [PubMed] [Google Scholar]
- Blom CWPM. Adaptations to flooding stress: from plant community to molecule. Plant Biology. 1999;1:261–273. [Google Scholar]
- Chen THH, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current Opinion in Plant Biology. 2002;5:250–257. doi: 10.1016/s1369-5266(02)00255-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Huber H, de Kroon H, et al. Intraspecific variation in the magnitude and pattern of flooding-induced shoot elongation in Rumex palustris. Annals of Botany. 2009;104:1057–1067. doi: 10.1093/aob/mcp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Visser EJW, de Kroon H, Pierik R, Voesenek LACJ, Huber H. Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytologist. 2011;190:409–420. doi: 10.1111/j.1469-8137.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AK, Kim J-K, Owens TG, et al. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proceedings of the National Academy of Sciences of the USA. 2002;99:15898–15903. doi: 10.1073/pnas.252637799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld HW, Voesenek LACJ. Submergence-induced petiole elongation in Rumex palustris is controlled by developmental stage and storage compounds. Plant and Soil. 2003;253:115–123. [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiology. 1995;108:735–741. doi: 10.1104/pp.108.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y. A major QTL confers rapid internode elongation in response to water rise in deepwater rice. Breeding Science. 2007;57:305–314. [Google Scholar]
- Hattori Y. Mapping of three QTLs that regulate internode elongation in deepwater rice. Breeding Science. 2008;58:39–46. [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Ethylene and responses of plants to soil waterlogging and submergence. Annual Review of Plant Physiology. 1985;36:145–174. [Google Scholar]
- Jackson MB, Ram PC. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Annals of Botany. 2003;91:227–241. doi: 10.1093/aob/mcf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsell B. Studies in the North-West European species of Rorippa s. str. Symbolae Botanicae Upsalienses. 1968;19:1–221. [Google Scholar]
- Keddy PA. Plant zonation on lakeshores in Nova Scotia: a test of the resource specialization hypothesis. Journal of Ecology. 1984;72:797–808. [Google Scholar]
- Kende H, van der Knaap E, Cho H-T. Deepwater rice: a model plant to study stem elongation. Plant Physiology. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesová J, Klimes L. Bud banks and their role in vegetative regeneration: a literature review and proposal for simple classification and assessment. Perspectives in Plant Ecology, Evolution and Systematics. 2007;8:115–129. [Google Scholar]
- Klimesova J, Kocianova A, Martinkova J. Weeds that can do both tricks: vegetative versus generative regeneration of the short-lived root-sprouting herbs Rorippa palustris and Barbarea vulgaris. Weed Research. 2008;48:131–135. [Google Scholar]
- Kudahettige NP, Pucciariello C, Parlanti S, Alpi A, Perata P. Regulatory interplay of the Sub1A and CIPK15 pathways in the regulation of α-amylase production in flooded rice plants. Plant Biology. 2011;13:611–619. doi: 10.1111/j.1438-8677.2010.00415.x. [DOI] [PubMed] [Google Scholar]
- Lenssen JPM, De Kroon H. Abiotic constraints at the upper boundaries of two Rumex species on a freshwater flooding gradient. Journal of Ecology. 2005;93:138–147. [Google Scholar]
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods in Molecular Biology. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiology. 2005;137:1130–1138. doi: 10.1104/pp.104.057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milly PCD, Wetherald RT, Dunne KA, Delworth TL. Increasing risk of great floods in a changing climate. Nature. 2002;415:514–517. doi: 10.1038/415514a. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T. Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution. Trends in Ecology and Evolution. 2001;16:693–700. [Google Scholar]
- Mommer L, Visser EJW. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany. 2005;96:581–589. doi: 10.1093/aob/mci212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Lenssen JPM, Huber H, Visser EJW, De Kroon H. Ecophysiological determinants of plant performance under flooding: a comparative study of seven plant families. Journal of Ecology. 2006a;94:1117–1129. [Google Scholar]
- Mommer L, Pons TL, Visser EJW. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. Journal of Experimental Botany. 2006b;57:283–290. doi: 10.1093/jxb/erj015. [DOI] [PubMed] [Google Scholar]
- Normile D. Reinventing rice to feed the world. Science. 2008;321:330–333. doi: 10.1126/science.321.5887.330. [DOI] [PubMed] [Google Scholar]
- Perata P, Alpi A. Plant responses to anaerobiosis. Plant Science. 1993;93:1–17. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. Anaerobic carbohydrate metabolism in wheat and barley, two anoxia-intolerant cereal seeds. Journal of Experimental Botany. 1996;47:999–1006. [Google Scholar]
- Pierik R, van Aken JM, Voesenek LACJ. Is elongation-induced leaf emergence beneficial for submerged Rumex species? Annals of Botany. 2009;103:353–357. doi: 10.1093/aob/mcn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram PC, Singh BB, Singh AK, et al. Submergence tolerance in rainfed lowland rice: physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Research. 2002;76:131–152. [Google Scholar]
- Raskin I. A method for measuring leaf volume, density, thickness, and internal gas volume. Alexandria, VA, USA: American Society for Horticultural Science; 1983. [Google Scholar]
- Raskin I, Kende H. Effect of submergence on translocation, starch content and amylolytic activity in deep-water rice. Planta. 1984;162:556–559. doi: 10.1007/BF00399922. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbour, NY: CSHL Press; 2001. [Google Scholar]
- Sauter M. Rice in deep water: ‘how to take heed against a sea of troubles. Naturwissenschaften. 2000;87:289–303. doi: 10.1007/s001140050725. [DOI] [PubMed] [Google Scholar]
- Schranz ME, Song B-H, Windsor AJ, Mitchell-Olds T. Comparative genomics in the Brassicaceae: a family-wide perspective. Current Opinion in Plant Biology. 2007;10:168–175. doi: 10.1016/j.pbi.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Setter TL, Laureles EV. The beneficial effect of reduced elongation growth on submergence tolerance of rice. Journal of Experimental Botany. 1996;47:1551–1559. [Google Scholar]
- Silvertown J, Dodd ME, Gowing DJG, Mountford JO. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature. 1999;400:61–63. [Google Scholar]
- Singh S, Mackill DJ, Ismail AM. Responses of SUB1 rice introgression lines to submergence in the field: Yield and grain quality. Field Crops Research. 2009;113:12–23. [Google Scholar]
- Slater R. The extraction of total RNA by the detergent and phenol method. In: Walker JM, editor. Methods in molecular biology. Vol. 2. Nucleic acids. Heidelberg: Springer; 1984. pp. 101–108. [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nature Protocols. 2006;1:1342–1345. doi: 10.1038/nprot.2006.232. [DOI] [PubMed] [Google Scholar]
- Sosnova M, van Diggelen R, Klimesova J. Distribution of clonal growth forms in wetlands. Aquatic Botany. 2010;92:33–39. [Google Scholar]
- Stift M, Luttikhuizen PC, Visser EJW, Van Tienderen PH. Different flooding responses in Rorippa amphibia and Rorippa sylvestris, and their modes of expression in F1 hybrids. New Phytologist. 2008;180:229–239. doi: 10.1111/j.1469-8137.2008.02547.x. [DOI] [PubMed] [Google Scholar]
- Stift M, Bregman R, Oostermeijer JGB, van Tienderen PH. Other tetraploid species and conspecific diploids as sources of genetic variation for an autotetraploid. American Journal of Botany. 2010;97:1858–1866. doi: 10.3732/ajb.1000048. [DOI] [PubMed] [Google Scholar]
- Thomson CJ, Armstrong W, Waters I, Greenway H. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant, Cell & Environment. 1990;13:395–403. [Google Scholar]
- Van Eck WHJM, Van De Steeg HM, Blom CWPM, De Kroon H. Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 terrestrial grassland species. Oikos. 2004;107:393–405. [Google Scholar]
- Van Leur H, Raaijmakers CE, Van Dam NM. Reciprocal interactions between the cabbage root fly (Delia radicum) and two glucosinolate phenotypes of Barbarea vulgaris. Entomologia Experimentalis et Applicata. 2008;128:312–322. [Google Scholar]
- Vashisht D, Hesselink A, Pierik R, et al. Natural variation of submergence tolerance among Arabidopsis thaliana accessions. New Phytologist. 2011;190:299–310. doi: 10.1111/j.1469-8137.2010.03552.x. [DOI] [PubMed] [Google Scholar]
- Vervuren PJA, Blom CWPM, de Kroon H. Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. Journal of Ecology. 2003;91:135–146. [Google Scholar]
- Voesenek L, van der Sman AJM, Harren FJM, Blom C. Ethylene and flooding resistance: an integration of plant hormone physiology and plant ecology. Chemical Regulation of Plants. 1991;26:156–172. [Google Scholar]
- Voesenek L, Banga M, Rijnders J, Visser EJW, Blom C. Hormone sensitivity and plant adaptations to flooding. Folia Geobotanica. 1996;31:47–56. [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, et al. Interactions between plant hormones regulate submergence induced shoot elongation in the flooding tolerant dicot Rumex palustris. Annals of Botany. 2003;91:205–211. doi: 10.1093/aob/mcf116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. How plants cope with complete submergence. New Phytologist. 2006;170:213–226. doi: 10.1111/j.1469-8137.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- Vreeburg RAM, Benschop JJ, Peeters AJM, et al. Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. The Plant Journal. 2005;43:597–610. doi: 10.1111/j.1365-313X.2005.02477.x. [DOI] [PubMed] [Google Scholar]
- Xu K. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.