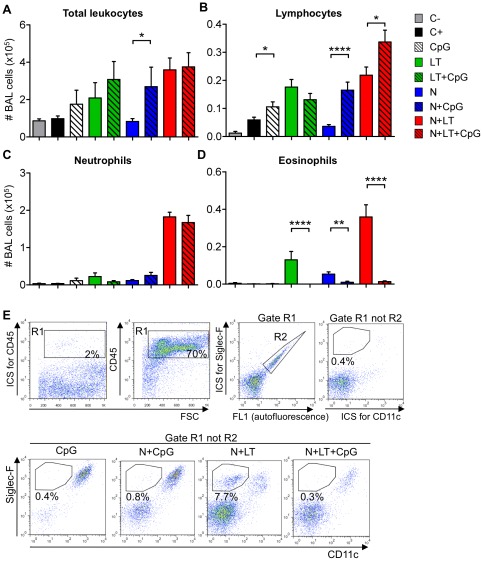
Figure 4. CpG added to the neonatal vaccine abrogated BAL eosinophilia upon RSV challenge.
Mice vaccinated as neonates as in Fig.1, with or without addition of 2 nmoles CpG (ODN 1826), as indicated, were sacrificed 5 days after the viral challenge with hRSV-A2. BAL leukocytes were numerated, cytocentrifuged and stained with May-Grünwald-Giemsa (MGG). Data are mean±SEM from n≥10 mice per group combining three independant experiments. Mann-Whitney test was performed to compare the total number of leukocytes (A), as well as the number of lymphocytes (B), neutrophils (C) and eosinophils (D) (** p<0.01; *** p<0.001 and **** p<0.0001). (E) BAL cells from each experimental group were pooled (n≥5 mice per group) and stained for FACS analysis using Siglec-F-PE, CD45-PerCP and CD11c-Biotin monoclonal antibodies followed by Streptavidine APC. Isotype control stainings (ICS) were done with irrelevant isotype-matched antibodies on a pool of BAL cells from all experimental groups. After gating on CD45+ leukocytes (R1) and excluding autofluorescent cells (R2), eosinophils were detected as Siglec-F+ and CD11clow. Data analysis was performed using FlowJo software with at least 5.000 events in the (R1 not R2) gate. Dot plots represent one of two experiments with similar data.