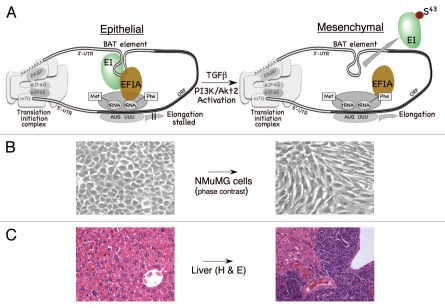
Figure 1.
TGFβ-activated translational (BAT) silencing mechanism mediating EMT and metastasis. (A) eEF 1A1 forms a complex with hnRNP E1 and the BAT element and silences translational elongation. Given the necessity for cognate-codon interaction with the ribosomal A site, it is likely that the formation of the BAT mRNP complex occurs post-delivery of the aminoacyl-tRNA to the ribosome. The ability of the BAT mRNP complex to inhibit eEF 1A1-dependent elongation suggests that the 3′-UT R is interacting with the 5′-UT R in a circularized model to facilitate its proximity to the 80S ribosome (left part). Following TGFβ-stimulation, activated Akt2 phosphorylates hnRNP E1 ar Ser43, which disrupts the mRNP complex and allows translation of Dab2 and ILEI mRNAs (right part). (B) Phase contrast image of control (left part) and NMuMG cells treated with TGFβ for 24 h (right part). TGFβ treatment causes distinct morphological changes associated with EMT, as discussed in the text. (C) Silencing of hnRNP E1 renders noninvasive NMuMG cells to form metastatic tumors in allograftic studies. H&E staining of liver tissue from mice injected with either NMuMG cells (left part) or SH14 cells (NMuMG cells harboring a stable shRNA against hnRNP E1) (right part).