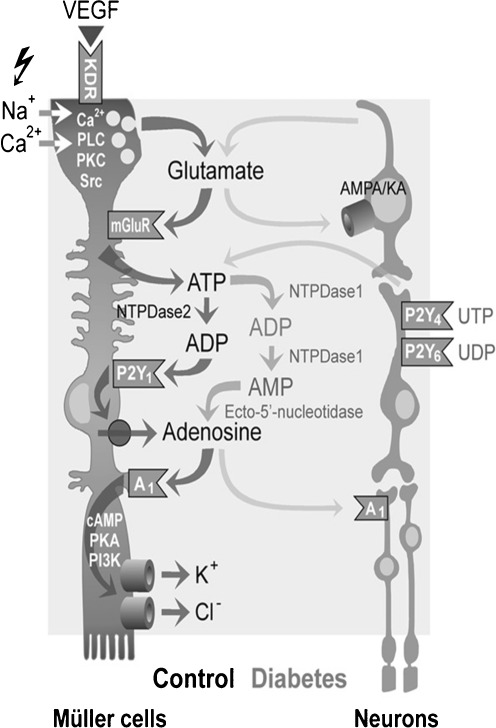
Fig. 3.
Modulation of retinal Müller glial cell functions and of their cross-talk with neurons by extracellular nucleotides and adenosine under control conditions and following diabetic retinopathy. Vascular endothelial growth factor (VEGF) evokes the exocytotic release of glutamate, which in turn activates metabotropic glutamate receptors (mGluRs) leading to the calcium-independent release of ATP from Müller cells. ATP is extracellularly converted by the nucleoside triphosphate diphosphohydrolase-2 to ADP that activates P2Y1, resulting in nucleoside transporter-mediated release of adenosine. Activation of A1 adenosine receptors causes the opening of potassium and chloride channels; the ion efflux equalizes the osmotic gradient across the plasma membrane and thus prevents water influx and cellular swelling under hypo-osmotic stress conditions. In swollen cells, the ion efflux is associated with a water efflux, resulting in decreased cell volume. Neuron-derived glutamate and ATP modulate the volume-regulatory signaling cascade depending upon neuronal activity. In the murine retina, activation of P2Y4,6 by UTP and UDP, respectively, might result in a release of neuronal ATP that activates glial P2Y1. Muller cell-derived glutamate and adenosine may also activate neuronal AMPA/KA and A1 receptors, resulting in stimulation and inhibition, respectively, of neuronal activity. In the retinal parenchyma of diabetic rats, increased extracellular adenosine concentrations further contributes to the swelling-inhibitory effect of glutamate and ATP. Reprinted and modified from Wurm et al. [108] with permission from Elsevier