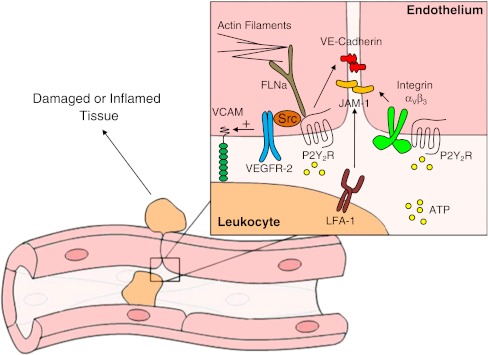
Fig. 4.
Proposed role of the endothelial P2Y2R in paracellular leukocyte diapedesis. Tissue damage or infection causes the local release of inflammatory signals that upregulate the endothelial P2Y2R as well as adhesion molecules required for leukocyte adhesion to vascular endothelium. These leukocytes begin migrating toward chemoattractants released from the damaged/infected site and, in so doing, release a burst of ATP from their leading edge [239]. We hypothesize that ATP released from adherent leukocytes activates the endothelial P2Y2R causing trafficking or relocation of this receptor to endothelial adherens junctions, possibly through interaction of the P2Y2R with Src, VEGFR-2, and the actin-binding protein, filamin A [22, 89, 100]. We also hypothesize that endothelial P2Y2R relocation to adherens junctions assists in the transient disruption of endothelial junctions and escorts leukocytes to endothelial borders for paracellular diapedesis, possibly through association of the P2Y2R with the αVβ3 integrin. This integrin is known to form a complex with the P2Y2R [23] and with JAM-1 [276], a junctional adhesion molecule in endothelial cells that interacts with the leukocyte integrin, LFA-1, and is important for both leukocyte adhesion and diapedesis [277]