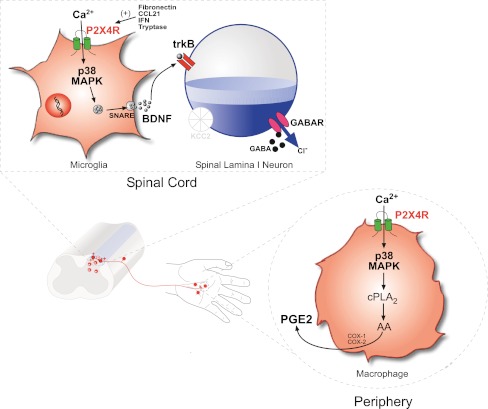
Fig. 1.
P2X4R-p38 MAPK is a core signaling pathway through which spinal microglia and peripheral macrophages contribute to neuropathic pain and inflammatory pain. a Following injury to a peripheral nerve, expression of P2X4R is upregulated in microglia that reside in the spinal dorsal horn. Several factors have recently been implicated in the upregulation of microglial P2X4R: the fibronectin-Lyn kinase signaling cascade, cytokine IFN-γ, tryptase released from mast cells, and the chemokine CCL21. Influx of Ca2+ through ATP stimulated P2X4R activates p38-MAPK and drives the synthesis and SNARE-dependent release of BDNF. Acting on its cognate receptor, trkB, BDNF released from microglia signals to dorsal horn lamina I neurons to downregulate the K+-Cl− cotransporter KCC2, resulting in aberrant nociceptive output that underlies pain hypersensitivity characterized by hyperalgesia, allodynia, and spontaneous pain. b Like microglia, peripheral macrophages constitutively express P2X4R. Inflammatory challenges that activate P2X4R on macrophages trigger Ca2+ influx and p38 MAPK phosphorylation. In macrophages, the P2X4R-p38 MAPK pathway is a signaling hub that activates cytosolic PLA2 which liberates arachidonic acid (AA) resulting in a cyclooxygenase (COX) dependent synthesis and release of prostaglandin E2 (PGE2), a key substrate that sensitizes primary sensory neurons and leads to hyperexcitability of peripheral nociceptive pathways that is a hallmark of inflammatory pain