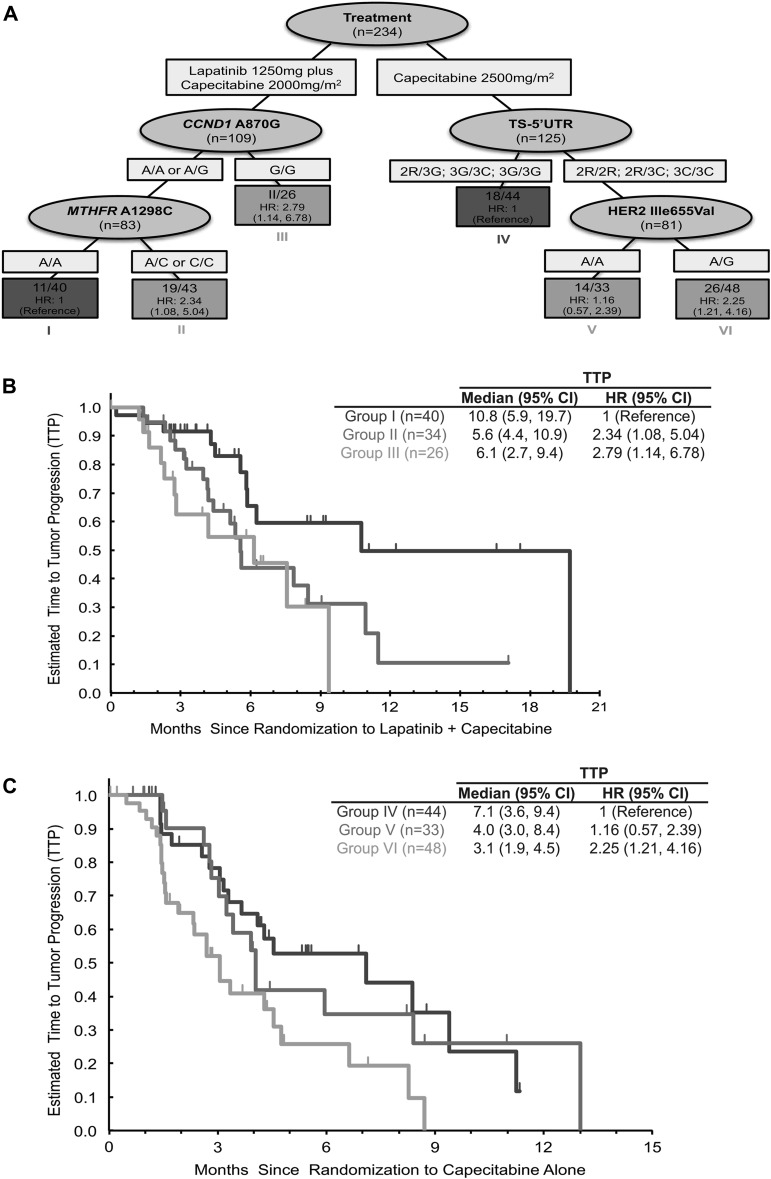
Figure 2.
Recursive partioning analysis. (A) This comprehensive recursive partitioning analysis for time to tumor progression (TTP) in metastatic breast cancer patients only incorporated a total of 18 potential markers to define six distinct patient groups (node 1–6) on the basis of TTP with treatment of capecitabine monotherapy or combination chemotherapy of lapatinib plus capecitabine. (B) Patients treated with lapatinib plus capecitabine that carry the CCND1 870 (rs17852153) A/A or A/G and the MTHFR 1298 (rs1801131) A/A genotype have a longer TTP (10.8 months) compared with those patients who carry the CCND1 870 A/A or A/G and MTHFR 1298 A/C or C/C genotypes [5.6 months, hazard ratio (HR) = 2.34, 95% confidence interval (CI) 1.08–5.054] or the CCND1 870 G/G genotype (6.1 months; HR = 2.79, 95% CI 1.14–6.78). (C) While patients who were treated with capecitabine alone carried the TS 5′UTR 2R/3G, 3G/3C or 3G/3G demonstrated a longer TTP of 7.1 months within this patient population compared with patients carrying the TS 5′UTR 2R/2R, 2R/3C or 3C/3C and either human epidermal receptor 2 655 A/G (3.1 months; HR = 2.25, 95% CI 1.21–4.16) or A/A (4.0 months; HR = 1.16, 95% CI 0.57–2.39) genotypes. CCND1, cyclin D1; MTHFR, methylenetetrahydrofolate reductase; TS, thymidylate synthase.