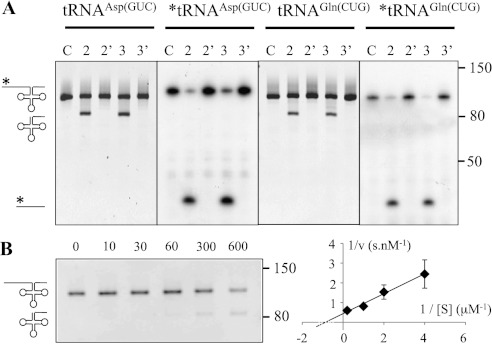
Figure 1.
The nuclear proteins PRORP2 and PRORP3 have RNase P activity. (A) The activity was assayed using in vitro transcripts representing the precursors of nuclear tRNAAsp(GUC) and tRNAGln(CUG). Precursor transcripts are 101 nt and 104 nt long, respectively. RNase P endonucleolytic cleavage results in the release of 5′ leader sequences 17 nt and 19 nt long, respectively. (Lanes C) Precursor transcripts alone. (Lanes 2,3) Precursor transcripts incubated with PRORP2 and PRORP3. (Lanes 2′,3′) Precursor transcripts incubated with the catalytic mutant of PRORP2 and PRORP3. After the reactions, RNA molecules were separated on 8% polyacrylamide gels and visualized by ethidium bromide staining and autoradiography. Stars show 5′ radiolabeled RNA molecules and autoradiographed gels. Molecular weights are given in nucleotides. (B) Kinetic analysis of tRNAGln cleavage by PRORP3. Representative experiment performed with 1 μM substrate for five reaction times (in seconds). Similar experiments were performed with four substrate concentrations. Average values from triplicate experiments are shown in a Lineweaver-Burk plot that was used to derive KM and vmax values.