Abstract
The α7* nicotinic acetylcholine receptor encoded by CHRNA7 (human)/Chrna7 (mice) regulates the release of both the inhibitory neurotransmitter γ-aminobutyric acid (GABA) and the excitatory neurotransmitter glutamate in the hippocampal formation. A heterozygous deletion at 15q13.3 containing CHRNA7 is associated with increased risk for schizophrenia, autism and epilepsy. Each of these diseases is characterized by abnormalities in excitatory and inhibitory hippocampal circuit function. Reduced Chrna7 expression results in decreased hippocampal α7* receptor density, abnormal hippocampal auditory sensory processing and increased hippocampal CA3 pyramidal neuron activity in C3H mice heterozygous for a null mutation in Chrna7. These abnormalities demonstrate that decreased Chrna7 expression alters hippocampal inhibitory circuit function. The current study examined the specific impact of reduced Chrna7 expression on hippocampal inhibitory circuits by measuring the levels of GABA, GABAA receptors, the GABA synthetic enzyme glutamate decarboxylase-65 (GAD-65) and the vesicular GABA transporter GAT-1 in wild type (Chrna7 +/+) and heterozygous (Chrna7 +/−) C3H α7 mice of both genders. GAD-65 levels were significantly decreased in male and female heterozygous C3H α7 mice while GABAA receptors were significantly reduced only in male heterozygous C3H α7 mice. No changes in GABA and GAT-1 levels were detected. These data suggest that reduced CHRNA7 expression may contribute to the abnormalities in hippocampal inhibitory circuits observed in schizophrenia, autism and/or epilepsy.
Keywords: Chrna7, λ-aminobutyric acid (GABA), GABAA receptors, hippocampus, nicotinic receptors, GAT-1
1.0
The α7* nicotinic acetylcholine receptor encoded by CHRNA7 (humans)/Chrna7 (mice) is a rapidly desensitizing, ligand-gated ion channel that fluxes calcium (Albuquerque et al., 2009, Bertrand, 2010). In hippocampus, the receptor is localized to neurons containing the inhibitory neurotransmitter γ-aminobutyric acid (GABA) as well as to those containing the excitatory neurotransmitter glutamate (Fabian-Fine et al., 2001, Albuquerque et al., 2009). Hippocampal α7* receptor activation leads to the release of both GABA and glutamate (Albuquerque et al., 2009), indicating that the receptor can modulate the inhibitory/excitatory balance of the hippocampus.
CHRNA7 expression appears to be abnormal in several neuropsychiatric disorders. A heterozygous microdeletion of chromosome 15q13.3 that encompasses several genes, including CHRNA7, is associated with increased risk for schizophrenia, autism and epilepsy (Helbig et al., 2009). Individuals with the microdeletion may exhibit no abnormalities or may exhibit a variety of clinical features, including cognitive deficits (developmental delay, learning disabilities, mild to severe mental retardation), behavioral problems (poor attention span, aggressive behavior, impulsivity, hyperactivity, psychosis, rage), neurologic anomalies (seizures, abnormal electroencephalograms, hypotonia), facial dysmorphia (large ears, bulbous nasal tip, prominent philtrum, everted upper lip, upslanting palpebral fissures), abnormal extremities (tapered fingers, 5th finger clinodactyly, lax thumb joint, brachydactyly) and/or cardiac defects (tetralogy of Fallot, cardiac hypoplasia, tricuspid stenosis, mitral valve prolapse) (van Bon et al., 2009, Masurel-Paulet et al., 2010, Cubells et al., 2011). CHRNA7 itself has been associated with the P50 gating and memory deficits observed in schizophrenic individuals as well as with schizophrenia in many, but not all, studies (Stephens et al., 2012). In addition, CHRNA7 has been associated with bipolar disease (Ancin et al., 2010) and with juvenile myoclonic epilepsy (Taske et al., 2002).
Schizophrenia, autism and epilepsy are characterized by abnormal excitatory and inhibitory circuits in numerous brain regions (Benes et al., 1996, Benes and Berretta, 2001, Volk and Lewis, 2002, Schleimer et al., 2004, Lee et al., 2006, Page et al., 2006, Eid et al., 2008, Sperk et al., 2009, Heckers and Konradi, 2010, Beneyto et al., 2011, Blatt and Fatemi, 2011). The density of α7* receptors is significantly decreased in several regions of postmortem brain from schizophrenic patients (Adams and Stevens, 2007). Similarly, α7* receptor mRNA is significantly lower in postmortem cortical samples from autistic individuals (Yasui et al., 2011). We hypothesize that reduced CHRNA7 expression contributes to the alterations in brain function observed in these disorders.
This hypothesis is supported by data from an animal model of reduced Chrna7 expression. Heterozygous (Het - Chrna7+/−) C3H α7 mice exhibit significant decreases in hippocampal α7* receptors, significant increases in hippocampal CA3 pyramidal neuron activity and abnormal hippocampal auditory sensory processing as compared to wild type (WT - Chrna7+/+) C3H α7 mice (Adams et al., 2008). These abnormalities demonstrate that decreased Chrna7 expression alters hippocampal inhibitory circuit function.
To determine the specific changes in hippocampal inhibitory circuits associated with reduced Chrna7 expression, we investigated the effects of reduced Chrna7 expression on hippocampal levels of GABA, the GABA synthetic enzyme L-glutamic acid decarboxylase-65 (GAD-65), the vesicular GABA transporter GAT-1 and GABAA receptors in adult WT and Het C3H α7 mice of both sexes. Examining WT C3H α7 mice allows for characterization of normal hippocampal levels of the GABAergic markers, whereas Het C3H α7 mice provide a model of the reduced CHRNA7 expression observed in some human diseases (e.g. schizophrenia and autism). Hippocampal GABA levels were measured to determine whether reduced Chrna7 expression alters baseline GABA production. The levels of hippocampal GAD-65, GAT-1 and GABAA receptors were examined because these markers are changed in individuals with schizophrenia, epilepsy and/or autism (Benes et al., 1996, Benes and Berretta, 2001, Volk and Lewis, 2002, Schleimer et al., 2004, Lee et al., 2006, Sperk et al., 2009, Blatt and Fatemi, 2011). Data from the present study indicate that reduced Chrna7 expression alters hippocampal GABAergic markers in a gender-specific manner.
2.0 Experimental Procedures
2.1 Animals
Mice were originally obtained from the Institute for Behavioral Genetics breeding colony at the University of Colorado, Boulder, CO. The C3H/Ibg (C3H) α7 null mutant (knockout - KO) mice were generated by backcrossing α7 KO mixed background (129 x C57BL/6) mice (Orr-Urtreger et al., 1997) to C3H mice (both sexes used) for 10 generations (maintaining 6 families). Mice from the last generation were used to establish a breeding colony in the animal facility of the Denver Veterans Administration Medical Center, Denver, CO. A recent genome diversity analysis (The Jackson Laboratory, Bar Harbor, Maine) indicates that the KO C3H α7 mice are at least 99.7% identical to the C3H parent strain.
Male and female WT and Het C3H α7 mice used in the current experiments were obtained from Het X Het matings at the VA animal facility. The pregnant dams were housed in large, low-air-flow cages with microisolator tops, Nestletts (Ancare, Bellmore, NY) and plastic igloos. The pups were weaned at 21 days and maintained in small low-air-flow cages with microisolator tops (5 mice/cage or less) and plastic igloos until collection at 5 – 6 months of age. Cage changes occurred twice weekly for all animals. All animals were provided with ad libitum water and Teklad rodent chow (Harlan Teklad, Indianapolis, ID) and a 12 hr light/dark cycle (lights on at 6 AM).
2.2 Tissue collection for GABAA receptor autoradiography
Adult (5 – 6 months of age) male and female WT and Het C3H α7 mice (n = 6/group) were rapidly decapitated without anesthesia, the brains were removed, frozen in dry ice snow and stored at −80°C until processing. Sequential cryostat sections (12 μm) through the hippocampus from animals of each genotype and gender were cut, thaw-mounted onto gelatin-coated slides and stored at −80°C before binding with the convulsant [35S]-tert-butylbicyclophosphorothionate ([35S]-TBPS, Perkin Elmer, Waltham, MA), an antagonist that binds to the chloride ionophore of GABAA receptors (Olsen et al., 1990).
2.3 Radioligand binding
The tissue, subdivided into total- and nonspecific-binding groups, was incubated in a solution containing 50 mM Tris-HCl and 0.9% sodium chloride (TBS buffer, pH 7.4, Sigma-Aldrich, St. Louis, MO) for 30 minutes at 4°C. Nonspecific binding was determined by adding 100 μM picrotoxin (Sigma-Aldrich, St. Louis, MO) to the TBS buffer. The two tissue sets were then incubated in the TBS buffer containing [35S]-TBPS (10 nM) at room temperature for 2 hours. The buffer for the nonspecific group again contained 100 μM picrotoxin. Following incubation with the ligand, the tissue was rinsed in TBS buffer for 3 × 5 min at 4°C. Subsequently, the slides were dipped briefly in cold distilled water, dried quickly under a stream of cool air and exposed to radiation-sensitive Hyperfilm (Amersham, Piscataway, NJ) for 72 hours with [14C] standards (Amersham, Piscataway, NJ) of known radioactivity in order to generate autoradiograms for quantitative analysis. Digital images were captured using a Chroma-Pro 45 light box and Retiga CCD camera (Qimaging, Surrey, BC, Canada) using Simple PCI software (Hamamatsu Corp., Sewickley, PA). Autoradiograms were quantified with a computer-based image analysis system (ImageJ, NIH) using calibrated standards of reference. A calibration curve of gray value against radioligand concentration (nCi/gm tissue) was constructed using standards of known radioactivity. Specific radioligand binding was calculated by subtracting values obtained in the presence of an excess of competing ligand (nonspecific binding) from those in the absence (total binding), and was expressed as nCi/g tissue. For each animal, measurements were taken from both left and right hemispheres of 15 hippocampal sections. [35S]-TBPS binding was minimal in stratum pyramidale and lucidum, so measurements of GABAA receptor density were not made in these layers.
2.4 Immunohistochemistry
Adult (5 – 6 months of age) male and female WT and Het C3H α7 mice (n = 6/group) were deeply anesthetized with Isoflurane and transcardially perfused with saline followed by a 4% paraformaldehyde/0.1M phosphate buffer (pH 7.4) fixative solution. The brains were removed, postfixed in the fixative for 6 hours, cryoprotected overnight in 30% sucrose/0.1 M phosphate buffer (pH 7.4), embedded in mounting media (Neg 50, Fisher Scientific, Pittsburg, PA), frozen in liquid nitrogen-chilled isopentane and stored at −80°C until processing. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
2.5 Antibodies
GAD-65: primary antibody (rabbit, 1:2000 dilution, Millipore Corp., Billerica, MA), donkey anti-rabbit secondary antibody (1:500, Jackson ImmunoResearch, West Grove, PA). GABA: primary antibody (guinea pig, 1:2000, Protos Biotech Corp., New York, NY), donkey anti-guinea pig secondary antibody (1:500, Jackson ImmunoResearch, West Grove, PA). GAT-1: primary antibody (rabbit, 1:100 dilution, Millipore Corp., Billerica, MA), donkey anti-rabbit secondary antibody (1:500, Jackson ImmunoResearch, West Grove, PA).
2.6 General immunohistochemistry procedure
Sequential cryostat sections (40 μm) through the hippocampus from animals of each genotype and gender were cut and collected in 0.1 M phosphate-buffered saline (PBS, pH 7.4). The tissue sections were rinsed 3 × 5 minutes in PBS, quenched in 0.3% hydrogen peroxide/PBS solution at room temperature (RT) for 30 minutes, rinsed 3 × 5 minutes in PBS and incubated in blocking solution (PBS-X, 5.0% normal donkey serum in PBS containing 0.3% Triton-X 100) for 1 hour at RT. The tissue was then placed in incubation solution (PBS-X containing 0.5% normal donkey serum) containing the primary antibody overnight at 4°C. The tissue was rinsed 3 × 5 minutes in PBS at RT and placed in incubation solution containing the secondary antibody for 1 hour at RT. The tissue was rinsed 3 × 5 minutes in PBS at RT and placed in ABC solution (Vector Laboratories, Burlingame, CA) for 1 hour at RT. The tissue was rinsed 3 × 5 minutes in PBS at RT and reacted in a solution containing 0.0004% 3,3′-diaminobenzidine, 0.002% hydrogen peroxide in PBS for 20 minutes followed by 3 × 5 minute rinses in PBS at RT. The tissue sections were mounted on gelatin-coated slides, dried under a stream of cool air and coverslipped with Permount (Fisher Scientific, Pittsburg, PA). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Deletion of the primary antibody was used as a control. Digital images were captured and analyzed as described previously (2.3). A Kodak No. 2 photographic step-tablet (Kodak, Rochester, NY) was used to generate a relative optical density curve. The background level of immunostaining (defined as the gray value in the corpus callosum) was subtracted from the level of immunostaining (gray values) in different hippocampal layers to give a final gray value for each layer. The final gray value was converted to a relative optical density value using the equation generated from the step-tablet. For each animal, measurements were taken from both left and right hemispheres of 15 hippocampal sections.
2.7 Statistical analysis
Data were analyzed by a 3-way ANOVA assessing differences in binding or immunohistochemical staining across hippocampal layers, gender and genotype, with Fisher’s Protected Least Significant Differences a posterior analyses where appropriate.
3.0 Results
3.1 Reduced Chrna7 expression is associated with decreased hippocampal GAD-65
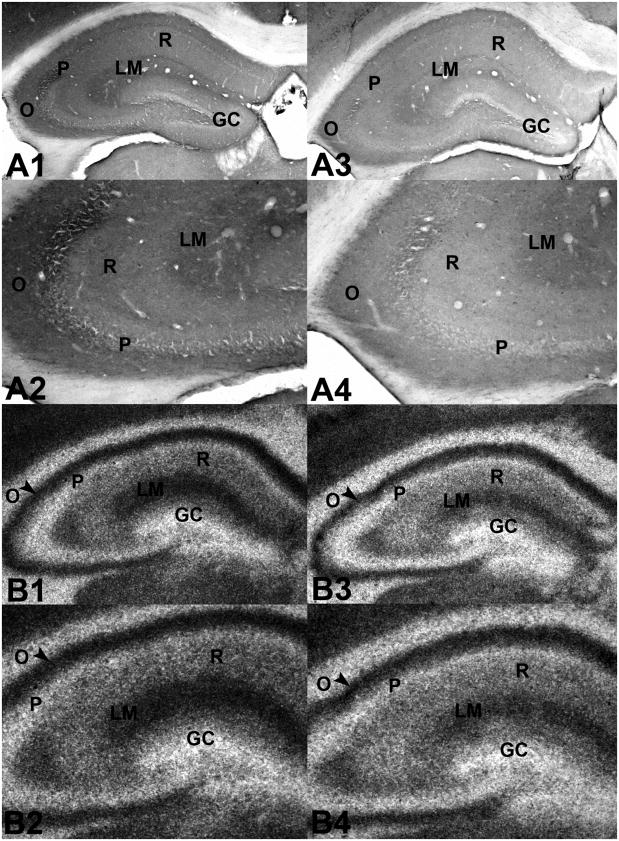
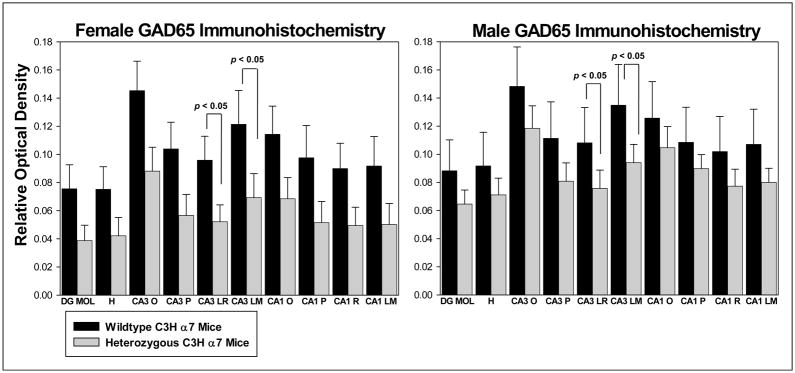
Immunostaining for GAD-65 was found predominately in neuronal terminals, although a few scattered GAD-65-positive cells were also observed (Figure 1A1–A4, Figure 3, Table 1). GAD-65 labeling was greatest in the outer molecular layer of the dentate gyrus, in stratum lacunosum/moleculare of hippocampal area CA3 and in stratum oriens of hippocampal areas CA3 and CA1 in both male and female WT and Het C3H α7 mice. Despite a similar pattern of staining, GAD-65 was generally decreased in the hippocampal formation of male and female Het mice compared to their WT counterparts (Table 1), although significant reductions were detected only in CA3 stratum lucidum/radiatum (F(1,20) = 3.855, p=0.042) and in CA3 stratum lacunosum/moleculare (F(1,20) = 3.855, p=0.043) (Figure 1B,C). Overall, even though no statistical differences in gender were detected, the Het-associated decrease in GAD-65 was greater in female (decreased 39 – 49%) than in male (decreased 17 – 30%) mice.
Figure 1.
Photomicrographs of transverse sections through the hippocampus of adult C3H α7 mice labeled for glutamate decarboxylase-65 (GAD-65) (A1 – A4) or the GABAA receptor antagonist [35S]-tert-butylbicyclophosphorothionate ([35S]-TBPS) (B1 – B4) A1. Transverse section through the hippocampal formation of a female wild type C3H α7 mouse immunolabeled for GAD-65. A2. Higher magnification of GAD-65 labeling in hippocampal area CA3 of the same section as in A1. A3. Transverse section through the hippocampal formation of a female heterozygous C3H α7 mouse immunolabeled for GAD-65. A4. Higher magnification of GAD-65 labeling in hippocampal area CA3 of the same section as in A3. Note the decrease in GAD-65 labeling in strata oriens, pyramidale, radiatum and lacunosum/moleculare of the heterozygous mouse (A3, A4) relative to the same regions in the wild type mouse (A1, A2). B1. Transverse section through the hippocampal formation of a male wild type C3H α7 mouse bound with the GABAA antagonist [35S]-TBPS. B2. Higher magnification of [35S]-TBPS binding in the same section as in B1. B3. [35S]-TBPS binding in a transverse section through the hippocampal formation of a male heterozygous C3H α7 mouse. B4. Higher magnification of [35S]-TBPS binding in the same section as in B3. Note the decrease in [35S]-TBPS binding in strata radiatum and lacunosum/moleculare of the heterozygous mouse (B3, B4) compared to the same regions in the wild type mouse (B1, B2). Abbreviations: GC - granule cell layer of the dentate gyrus, O - stratum oriens, P - stratum pyramidale, R - stratum radiatum, LM - stratum lacunosum/moleculare.
Figure 3.
Qualitative measurement of immunostaining for the GABA synthesizing enzyme GAD-65 in hippocampal sections from female (n = 6/group, left panel) and male (n = 6/group, right panel) wild type and heterozygous C3H a7 mice. Decreased levels of GAD-65 immunostaining were seen in both male and female heterozygous mice, with significant (p < 0.05) decreases in strata lucidum/radiatum and lacunosum/moleculare of hippocampal area CA3 in mice of both genders. Abbreviations: DG MOL – molecular layer of the dentate gyrus, H – hilus (polymorph layer) of dentate gyrus, CA3 O – oriens layer of hippocampal area CA3, CA3 P – pyramidal cell layer of hippocampal area CA3, CA3 LR – lucidum/radiatum layer of hippocampal area CA3, CA3 LM – lacunosum moleculare layer of hippocampal area CA3, CA1 O – oriens layer of hippocampal area CA1, CA1 P – pyramidal cell layer of hippocampal area CA1, CA1 R – radiatum layer of hippocampal area CA1, CA1 LM – lacunosum moleculare of hippocampal area CA1. Values = mean ± S.E.M.
Table 1.
Hippocampal GAD65 Levels in C3H α7 Wildtype vs Heterozygous Mice
| Layer | F(1,20) = | p |
|---|---|---|
| Dentate gyrus molecular layer | 3.617 | 0.072✠ |
| Dentate gyrus hilus | 2.471 | 0.132 |
| CA3 Oriens | 4.259 | 0.052✠ |
| CA3 Pyramidale | 4.297 | 0.051✠ |
| CA3 Lucidum/Radiatum | 4.698 | 0.042* |
| CA3 Lacunosum moleculare | 4.651 | 0.043* |
| CA1 Oriens | 2.935 | 0.102 |
| CA1 Pyramidale | 2.874 | 0.106 |
| CA1 Radiatum | 3.439 | 0.078✠ |
| CA1 Lacunosum moleculare | 3.373 | 0.081 |
Statistically significant
Trend
3.2 Reduced Chrna7 expression is associated with decreased hippocampal GABAA receptors in males
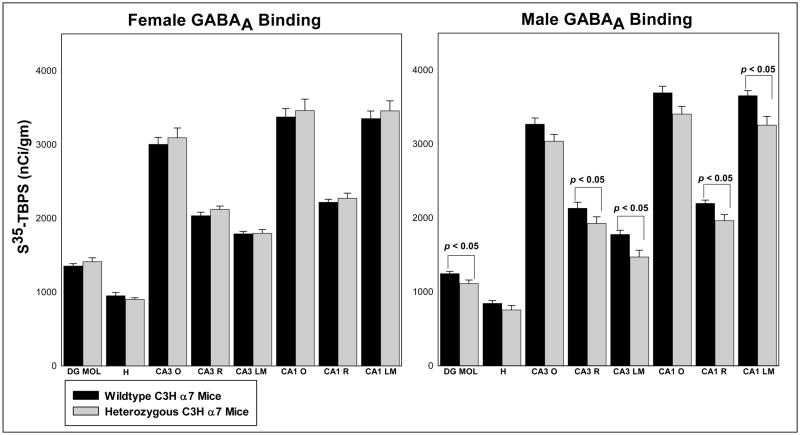
Binding of [35S]-TBPS was greatest in the outer moleculare layer of the dentate gyrus, in stratum oriens of hippocampal areas CA3 and CA1 and in stratum lacunosum/moleculare of hippocampal area CA1 in all mice (Figure 1B1–B4, Figure 4, Table 2). Lower levels of [35S]-TBPS binding were observed in stratum radiatum of areas CA3 and CA1 and in the inner molecular layers and hilus of the dentate gyrus. Binding was virtually absent in the granule cell layer of the dentate gyrus, in strata pyramidale and lucidum of hippocampal area CA3 and in stratum pyramidale of hippocampal area CA1. Again, although the pattern of binding was comparable, GABAA receptor density was significantly decreased in the molecular layer of the dentate gyrus (F(1,20) = 5.165, p=0.034), in CA3 stratum radiatum (F(1,20) = 4.426, p=0.048) and stratum lacunosum/moleculare (F(1,20) = 6.314, p=0.021) and in CA1 stratum radiatum (F(1,20) = 5.485, p=0.030) and stratum lacunosum/moleculare (F(1,20) = 5.245, p=0.033) of male Het compared to male WT mice (Table 2). The decrease in binding in the male Het mice ranged from 7 – 17%. In contrast, GABAA receptor density was comparable in the hippocampal formation of female WT and Het C3H α7 mice. The subunit composition and location (presynaptic, postsynaptic and/or extrasynaptic) of the affected GABAA receptors is currently unknown.
Figure 4.
[35S]-TBPS binding in hippocampal sections from female (n = 6/group, left panel) and male (n = 6/group, right panel) wildtype and heterozygous C3H a7 mice. No significant differences in GABAA receptor levels were seen in the female mice. In contrast, significant decreases in GABAA receptor density were seen in the molecular layer of the dentate gyrus and in strata radiatum and lacunosum moleculare of hippocampal areas CA3 and CA1 in male heterozygous C3H a7 mice. Abbreviations: DG MOL – molecular layer of the dentate gyrus, H – hilus (polymorph layer) of dentate gyrus, CA3 O – oriens layer of hippocampal area CA3, CA3 R – radiatum layer of hippocampal area CA3, CA3 LM – lacunosum moleculare layer of hippocampal area CA3, CA1 O – oriens layer of hippocampal area CA1, CA1 R – radiatum layer of hippocampal area CA1, CA1 LM – lacunosum moleculare of hippocampal area CA1. Values = mean ± S.E.M.
Table 2.
GABAA Receptor Density in the Hippocampal Formation of Male and Female C3H α7 Wildtype and Heterozygous Mice
| Layer | Gender F(1,20)= | p | Genotype F(1,20)= | p | Interaction F(1,20)= | p |
|---|---|---|---|---|---|---|
| Dentate gyrus of the molecular layer | 23.125 | <0.001 | 0.778 | 0.388 | 5.165 | 0.034 |
| Dentate gyrus hilus | 8.270 | 0.009 | 2.538 | 0.127 | 0.190 | 0.667 |
| CA3 Oriens | 1.048 | 0.318 | 0.471 | 0.500 | 2.438 | 0.134 |
| CA3 Radiatum | 0.525 | 0.477 | 0.731 | 0.403 | 4.426 | 0.048 |
| CA3 Lacunosum moleculare | 7.466 | 0.013 | 5.676 | 0.027 | 6.314 | 0.021 |
| CA1 Oriens | 1.238 | 0.279 | 0.737 | 0.401 | 2.527 | 0.128 |
| CA1 Radiatum | 7.304 | 0.014 | 2.141 | 0.159 | 5.485 | 0.030 |
| CA1 Lacunosum moleculare | 0.200 | 0.660 | 1.792 | 0.196 | 5.245 | 0.033 |
3.3 Reduced Chrna7 expression does not alter hippocampal GABA levels
Scattered GABA-positive neurons were observed throughout the hippocampal formation (Figure 2A1–A2). In addition, somewhat higher levels of diffuse staining were seen in the hilus of the dentate gyrus, in stratum lacunosum/moleculare of hippocampal area CA3, at the border of stratum radiatum and lacunosum/moleculare of hippocampal area CA1 and in stratum pyramidale of areas CA3 and CA1. No significant differences in GABA levels were found between genotypes or genders (data not shown).
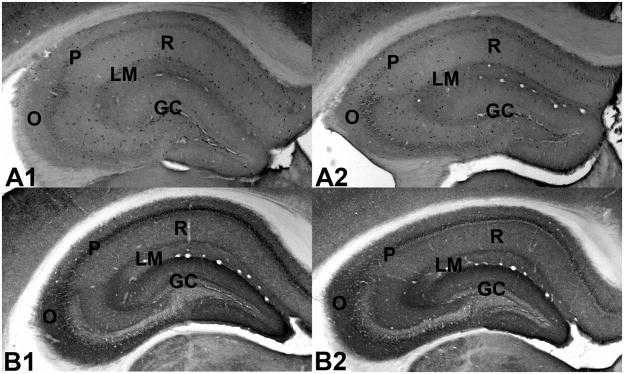
Figure 2.
Photomicrographs of transverse sections through the hippocampus of adult C3H α7 mice labeled for γ-aminobutyric acid (GABA) (A1, A2) or the GABA vesicular transporter GAT-1 (B1, B2). A1. Transverse section through the hippocampal formation of a male wild type C3H α7 mouse labeled for GABA. A2. Transverse section through the hippocampal formation of a male heterozygous C3H α7 mouse labeled for GABA. The level of GABA labeling is comparable between the two genotypes. B1. Transverse section through the hippocampal formation of a male wild type C3H α7 mouse labeled for GAT-1. B2. Transverse section through the hippocampal formation of a male heterozygous C3H α7 mouse labeled for GAT-1. The level of GAT-1 labeling is comparable between the two genotypes. Abbreviations: GC - granule cell layer of the dentate gyrus, O - stratum oriens, P - stratum pyramidale, R - stratum radiatum, LM - stratum lacunosum/moleculare.
3.4 Reduced Chrna7 expression does not alter hippocampal GAT-1 levels
GAT-1 labeling was restricted to neuronal terminals and was highest in the outer molecular layer of the dentate gyrus, in stratum lacunosum/moleculare of hippocampal area CA3 and in stratum pyramidale of hippocampal areas CA3 and CA1 (Figure 2B1–B2). Somewhat lower levels of GAT-1 were seen in strata oriens and radiatum of hippocampal area CA3 and in stratum lacunosum/moleculare of hippocampal area CA1. The lowest levels of GAT-1 were observed in strata oriens and radiatum of hippocampal area CA1. No significant differences in GAT-1 levels were observed between genotypes or genders (data not shown).
4.0 Discussion
Hippocampal GAD-65 was significantly reduced in Het C3H α7 mice of both genders while hippocampal GABAA receptors were significantly reduced in male Het mice only. No significant differences in GABA or GAT-1 levels were detected. These data suggest that reduced Chrna7 expression may alter some, but not all, hippocampal inhibitory circuit components in a gender-specific manner.
4.1 Reduced GAD-65
GAD-65 is primarily located in inhibitory nerve terminals (Martin and Tobin, 2000). Therefore, the lower GAD-65 levels observed in Het C3H α7 mice could indicate that reduced Chrna7 expression leads to a decrease in hippocampal inhibitory terminal density. However, the lack of change in hippocampal GAT-1, also an inhibitory terminal marker (Zahniser and Doolen, 2001), does not support this supposition.
Another possibility is that altered kinase function may underlie the observed decrease in GAD-65. Expression of Gad2, the gene encoding GAD-65, is regulated by the phosphorylated form of cAMP response element binding protein (pCREB) (Bitner et al., 2007, Gubbins et al., 2010). In addition, GAD-65 activity is increased by phosphorylation via protein kinase C (PKC) and possibly by Ca2+-calmodulin-dependent protein kinase II (CaMKII) and mitogen-activated protein kinase (MAPK) (Wei et al., 2004, Wei and Wu, 2008). Stimulation of the α7* receptor activates CaMKII, MAPK and PKC (Zhang and Berg, 2007; Wanaverbecq et al., 2007) and CaMKII and MAPK activation secondary to α7* receptor stimulation increases pCREB levels in hippocampal neurons (Hu et al., 2002). Therefore, α7* receptor stimulation may modulate hippocampal Gad2 expression and/or GAD-65 activation by increasing kinase function. If so, a decrease in hippocampal α7* receptor density could lead to reduced kinase activation and/or decreased Gad2 expression, resulting in lower GAD-65 levels.
GAD-65 levels were reduced 20 – 47% in postmortem hippocampus from unmedicated schizophrenic individuals relative to controls (Benes and Berretta, 2001), a reduction comparable to the 17 – 49% decrease in GAD-65 observed in Het C3H α7 mice in the present study. GAD-65 contributes primarily to the activity-regulated synthesis and release of vesicular GABA (Martin and Tobin, 2000). A disruption of activity-dependent GABA release might shift the inhibitory/excitatory balance within the hippocampus to a more excited state. Such a shift could contribute to both the auditory sensory processing deficits and the hyperactivity observed in hippocampus from Het C3H α7 mice (Adams et al., 2008) and schizophrenic patients (Tregellas et al., 2007).
4.2 Reduced GABAA receptor density
Reduced Chrna7 expression was associated with decreased hippocampal GABAA receptor density in males. Although the anatomical (pre/postsynaptic) location of the decreased binding is not known, modest (5 – 35%) reductions in postsynaptic GABAA receptors are associated with significant behavioral alterations (Luscher et al., 2011). This suggests that the 7 – 17% decrease in hippocampal GABAA receptors observed in male Het mice in the current study may have negative functional consequences.
GABAA receptor density is regulated by a variety of mechanisms (Barnes, 1996, Luscher et al., 2011). Prolonged exposure to GABA or GABAergic agonists can cause a decrease in GABAA receptor levels (Barnes, 1996). However, as baseline GABA levels were not significantly different between Het and WT mice, it is unlikely that the decrease in GABAA receptor density observed in male Het mice was due to a ligand-mediated downregulation.
Another possibility is that α7* receptor-dependent changes in BDNF are responsible for reduced GABAA in male Het mice. Activation of tropomyosin related kinase-B (TrkB) receptors by BDNF leads to a decrease in hippocampal GABAA receptor density (Brunig et al., 2001, Wardle and Poo, 2003, Lund et al., 2008, Mou et al., 2011). Blockade of hippocampal α7* receptors by intracerebroventricular infusion of the α7* receptor-selective antagonist α-bungarotoxin increased BDNF mRNA throughout the hippocampal formation (Freedman et al., 1993). Therefore, reduced Chrna7 expression may set off a BDNF-mediated signaling cascade that results in reduced GABAA receptor levels.
A third possibility is that reduced GABAA receptor levels in the male Het mice may be mediated by changes in kinase function. Activation of CaMKII results in a rapid increase in GABAA receptor density (Marsden et al., 2007). As described above (4.2), α7* receptor stimulation activates CaMKII (Zhang and Berg, 2007). Therefore, the reduction in GABAA receptors observed in male Het C3H α7 mice could also occur secondary to reduced α7* receptor-mediated CaMKII activation.
Finally, we do not know to what extent gender differences in hippocampal GABAA receptor density might be due to gonadal steroid influences. As female mice were collected over a period of months, it seems likely that any estrus cycle influence on GABAA receptors would average out. However, if this was not the case, estrogen has been found to regulate hippocampal BDNF levels (Murphy et al., 1998, Scharfman et al., 2003, Spencer et al., 2008). Estrogen also modulates hippocampal acetylcholine release in females, but not males (Gabor et al., 2003). These two factors could contribute to the observed gender differences in hippocampal GABAA receptor density. In addition, the gender difference in GABAA receptor density could result from the action of neurosteroid metabolites of progesterone and/or testosterone as these metabolites act as positive allosteric modulators of GABAA receptors (Majewska, 1992, Gonzalez-Flores et al., 2004, Reddy and Jian, 2010).
In contrast to the current study, an increase in GABAA receptor binding density was observed in postmortem hippocampus from schizophrenic patients (Benes and Berretta, 2001). However, recent studies have reported significant decreases in mRNA for the α1 subunit of the GABAA receptor in prefrontal cortex of individuals with schizophrenia (Beneyto et al., 2011, Glausier and Lewis, 2011). An examination of potential GABAA receptor subunit alterations in the Het C3H α7 mice may help clarify this issue.
4.3 GABA and GAT-1 regulation
Baseline hippocampal GABA levels were not significantly different across mouse groups in the present study. Although not examined, this might be due to the maintenance of GABA levels by L-glutamic acid decarboxylase-67 (GAD-67) (Martin and Tobin, 2000). Normal hippocampal GABA levels in all mouse groups could account, at least in part, for the absence of change in hippocampal GAT-1 levels as GAT-1 is regulated by GABA (Bernstein and Quick, 1999).
4.4 Conclusion
In summary, reduced Chrna7 expression is associated with decreased hippocampal GAD-65 and GABAA receptor levels in C3H α7 mice. Lower GAD-65 may result in decreased activity-dependent GABA release which could, in turn, contribute to the hippocampal disinhibition and deficits in hippocampal auditory sensory processing observed in Het C3H α7 mice. The impact of reduced hippocampal GABAA receptor density in male Het mice is less clear. We also do not know when the alterations in hippocampal GABAA receptors and GAD-65 occur in the Het mice. The α7* receptor modulates a number of mechanisms important for hippocampal development (Maggi et al., 2001, Maggi et al., 2003, Liu et al., 2006) and does not reach mature levels in rodents until two months of age (Adams et al., 2002), raising the possibility that the GABAergic marker changes may occur prior to the adult age examined in this study. Although a great deal remains to be determined, these data suggest that reduced Chrna7 expression can alter hippocampal inhibitory function, perhaps contributing to the inhibitory deficits observed in schizophrenia, epilepsy and autism.
Hippocampal GAD-65 is significantly reduced in male and female heterozygous C3H α7 mice.
Hippocampal GABAA receptor density is significantly reduced in male heterozygous C3H α7 mice.
Hippocampal GABA is comparable between wildtype and heterozygous C3H α7 mice.
Hippocampal GAT-1 is comparable between wildtype and heterozygous C3H α7 mice.
Acknowledgments
Supported by a Veterans Administration Merit Review and Conte Center Award 5P50MH086383-03
Abbreviations
- ADNFLE
autosomal dominant nocturnal frontal lobe epilepsy
- BDNF
brain derived neurotrophic factor
- CaMKII
Ca2+-calmodulin-dependent protein kinase II
- CHRNA7/Chrna7
gene (human/mouse) encoding the α7 nicotinic acetylcholine receptor
- CREB
cAMP response element binding protein
- GABA
γ-aminobutyric acid
- Gad2
gene encoding GAD-65
- GAD-65
L-glutamic acid decarboxylase-65
- GAD-67
L-glutamic acid decarboxylase-67
- GAT-1
vesicular GABA transporter 1
- [3H]
tritiated
- Het
heterozygous
- KO
knockout
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- PBS-X
phosphate-buffered saline containing Triton-X 100
- PKC
protein kinase C
- RT
room temperature
- [35S]-TBPS
[35S]-tert-butylbicyclophosphorothionate
- TrkB
tropomyosin related kinase-B
- TBS
Tris-HCl/sodium chloride buffer
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Adams CE, Stevens KE. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front Biosci. 2007;12:4755–4772. doi: 10.2741/2424. [DOI] [PubMed] [Google Scholar]
- Adams CE, Yonchek JC, Zheng L, Collins AC, Stevens KE. Altered hippocampal circuit function in C3H alpha7 null mutant heterozygous mice. Brain Res. 2008;1194:138–145. doi: 10.1016/j.brainres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancin I, Barabash A, Vazquez-Alvarez B, Santos JL, Sanchez-Morla E, Martinez JL, Aparicio A, Pelaez JC, Diaz JA. Evidence for association of the non-duplicated region of CHRNA7 gene with bipolar disorder but not with Schizophrenia. Psychiatr Genet. 2010;20:289–297. doi: 10.1097/YPG.0b013e32833a9b7a. [DOI] [PubMed] [Google Scholar]
- Barnes EM., Jr Use-dependent regulation of GABAA receptors. Int Rev Neurobiol. 1996;39:53–76. doi: 10.1016/s0074-7742(08)60663-7. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996;22:338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cerebral cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein EM, Quick MW. Regulation of gamma-aminobutyric acid (GABA) transporters by extracellular GABA. J Biol Chem. 1999;274:889–895. doi: 10.1074/jbc.274.2.889. [DOI] [PubMed] [Google Scholar]
- Bertrand D. Neurocircuitry of the nicotinic cholinergic system. Dialogues Clin Neurosci. 2010;12:463–470. doi: 10.31887/DCNS.2010.12.4/dbertrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, Li J, Malysz J, Markosyan S, Marsh K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmermann D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27:10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Fatemi SH. Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat Rec (Hoboken) 2011;294:1646–1652. doi: 10.1002/ar.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. European Journal of Neuroscience. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Deoreo EH, Harvey PD, Garlow SJ, Garber K, Adam MP, Martin CL. Pharmaco-genetically guided treatment of recurrent rage outbursts in an adult male with 15q13.3 deletion syndrome. Am J Med Genet A. 2011;155A:805–810. doi: 10.1002/ajmg.a.33917. [DOI] [PubMed] [Google Scholar]
- Eid T, Williamson A, Lee TS, Petroff OA, de Lanerolle NC. Glutamate and astrocytes--key players in human mesial temporal lobe epilepsy? Epilepsia. 2008;49(Suppl 2):42–52. doi: 10.1111/j.1528-1167.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. Alpha-bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB. Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Res. 2003;962:244–247. doi: 10.1016/s0006-8993(02)04053-2. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Sanchez N, Garcia-Juarez M, Lima-Hernandez FJ, Gonzalez-Mariscal G, Beyer C. Estradiol and testosterone modulate the anesthetic action of the GABA-A agonist THIP, but not of the neurosteroid 3alpha,5beta-pregnanolone in the rat. Psychopharmacology (Berl) 2004;172:283–290. doi: 10.1007/s00213-003-1649-x. [DOI] [PubMed] [Google Scholar]
- Gubbins EJ, Gopalakrishnan M, Li J. Alpha7 nAChR-mediated activation of MAP kinase pathways in PC12 cells. Brain Res. 2010;1328:1–11. doi: 10.1016/j.brainres.2010.02.083. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, Franke A, Muhle H, de Kovel C, Baker C, von Spiczak S, Kron KL, Steinich I, Kleefuss-Lie AA, Leu C, Gaus V, Schmitz B, Klein KM, Reif PS, Rosenow F, Weber Y, Lerche H, Zimprich F, Urak L, Fuchs K, Feucht M, Genton P, Thomas P, Visscher F, de Haan GJ, Moller RS, Hjalgrim H, Luciano D, Wittig M, Nothnagel M, Elger CE, Nurnberg P, Romano C, Malafosse A, Koeleman BPC, Lindhout D, Stephani U, Schreiber S, Eichler EE, Sander T. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nature Genetics. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- Lee TS, Bjornsen LP, Paz C, Kim JH, Spencer SS, Spencer DD, Eid T, de Lanerolle NC. GAT1 and GAT3 expression are differently localized in the human epileptogenic hippocampus. Acta Neuropathol. 2006;111:351–363. doi: 10.1007/s00401-005-0017-9. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature “silent” connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DL, Tobin AJ. Mechanisms controlling GABA synthesis and degradation in the brain. In: Martin DL, ORW, editors. GABA in the Nervous System: The View at Fifty Years. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 25–41. [Google Scholar]
- Masurel-Paulet A, Andrieux J, Callier P, Cuisset JM, Le Caignec C, Holder M, Thauvin-Robinet C, Doray B, Flori E, Alex-Cordier MP, Beri M, Boute O, Delobel B, Dieux A, Vallee L, Jaillard S, Odent S, Isidor B, Beneteau C, Vigneron J, Bilan F, Gilbert-Dussardier B, Dubourg C, Labalme A, Bidon C, Gautier A, Pernes P, Pinoit JM, Huet F, Mugneret F, Aral B, Jonveaux P, Sanlaville D, Faivre L. Delineation of 15q13.3 microdeletions. Clinical Genetics. 2010;78:149–161. doi: 10.1111/j.1399-0004.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- Mou L, Heldt SA, Ressler KJ. Rapid brain-derived neurotrophic factor-dependent sequestration of amygdala and hippocampal GABA(A) receptors via different tyrosine receptor kinase B-mediated phosphorylation pathways. Neuroscience. 2011;176:72–85. doi: 10.1016/j.neuroscience.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, McCabe RT, Wamsley JK. GABAA receptor subtypes: autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J Chem Neuroanat. 1990;3:59–76. [PubMed] [Google Scholar]
- Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, Ambery F, McAlonan GM, Murphy KC, Murphy DG. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am J Psychiatry. 2006;163:2189–2192. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334:1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer SB, Hinton T, Dixon G, Johnston GA. GABA transporters GAT-1 and GAT-3 in the human dorsolateral prefrontal cortex in schizophrenia. Neuropsychobiology. 2004;50:226–230. doi: 10.1159/000079975. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Drexel M, Pirker S. Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia. 2009;50(Suppl 12):29–31. doi: 10.1111/j.1528-1167.2009.02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SH, Franks A, Berger R, Palionyte M, Fingerlin TE, Wagner B, Logel J, Olincy A, Ross RG, Freedman R, Leonard S. Multiple genes in the 15q13–q14 chromosomal region are associated with schizophrenia. Psychiatr Genet. 2012;22:1–14. doi: 10.1097/YPG.0b013e32834c0c33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taske NL, Williamson MP, Makoff A, Bate L, Curtis D, Kerr M, Kjeldsen MJ, Pang KA, Sundqvist A, Friis ML, Chadwick D, Richens A, Covanis A, Santos M, Arzimanoglou A, Panayiotopoulos CP, Whitehouse WP, Rees M, Gardiner RM. Evaluation of the positional candidate gene CHRNA7 at the juvenile myoclonic epilepsy locus (EJM2) on chromosome 15q13–14. Epilepsy Res. 2002;49:157–172. doi: 10.1016/s0920-1211(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon BW, Mefford HC, Menten B, Koolen DA, Sharp AJ, Nillesen WM, Innis JW, de Ravel TJ, Mercer CL, Fichera M, Stewart H, Connell LE, Ounap K, Lachlan K, Castle B, Van der Aa N, van Ravenswaaij C, Nobrega MA, Serra-Juhe C, Simonic I, de Leeuw N, Pfundt R, Bongers EM, Baker C, Finnemore P, Huang S, Maloney VK, Crolla JA, van Kalmthout M, Elia M, Vandeweyer G, Fryns JP, Janssens S, Foulds N, Reitano S, Smith K, Parkel S, Loeys B, Woods CG, Oostra A, Speleman F, Pereira AC, Kurg A, Willatt L, Knight SJ, Vermeesch JR, Romano C, Barber JC, Mortier G, Perez-Jurado LA, Kooy F, Brunner HG, Eichler EE, Kleefstra T, de Vries BB. Further delineation of the 15q13 microdeletion and duplication syndromes: a clinical spectrum varying from non-pathogenic to a severe outcome. J Med Genet. 2009;46:511–523. doi: 10.1136/jmg.2008.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Davis KM, Wu H, Wu JY. Protein phosphorylation of human brain glutamic acid decarboxylase (GAD)65 and GAD67 and its physiological implications. Biochemistry. 2004;43:6182–6189. doi: 10.1021/bi0496992. [DOI] [PubMed] [Google Scholar]
- Wei J, Wu JY. Post-translational regulation of L-glutamic acid decarboxylase in the brain. Neurochem Res. 2008;33:1459–1465. doi: 10.1007/s11064-008-9600-5. [DOI] [PubMed] [Google Scholar]
- Yasui DH, Scoles HA, Horike S, Meguro-Horike M, Dunaway KW, Schroeder DI, Lasalle JM. 15q11.2–13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum Mol Genet. 2011;20:4311–4323. doi: 10.1093/hmg/ddr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Doolen S. Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol Ther. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Berg DK. Reversible inhibition of GABA(A) receptors by alpha 7-containing nicotinic receptors on the vertebrate postsynaptic neurons. Journal of Physiology-London. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]