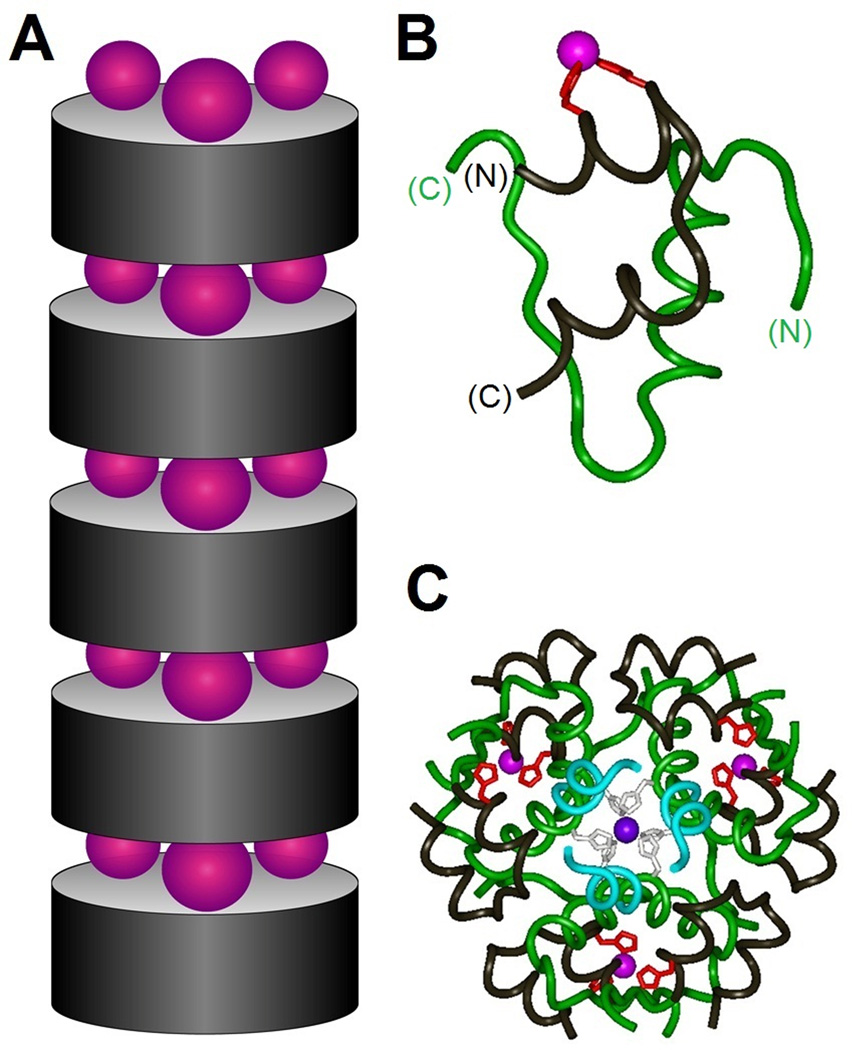
Figure 8.
Zinc-stapled insulin analogs. (A) Schematic model showing successive stacking of insulin hexamers (gray cylinders) bridged by layers of zinc ions (purple spheres). Upper and lower surfaces of insulin analog hexamer present three α-helix-related HX3H half-coordination sites; each site recapitulates the C-terminal half of the classical zinc finger (see Fig. 3). (B) Ribbon model of variant insulin monomer showing designed HX3H sites (red) at residue positions A4 (left) and A8 (right) in the N-terminal A-chain α-helix. The A chain is otherwise shown in black, and the B chain in green. (C) Crystal structure of variant R6 insulin hexamer. The three novel interfacial zinc ions are shown in magenta whereas the two classical axial zinc ions (coordinated by HisB10; black) are shown in purple (overlaid in center). The color code is otherwise as in Figure 1 with the A chain in black and B chain in blue (residues B1–B6) or green (residues B7–B30). In the crystal lattice successive insulin hexamers are stacked as in panel A with intervening layers of zinc ions.78 Coordinates were obtained from Protein Databank entry 3KQ6.