Figure 6.
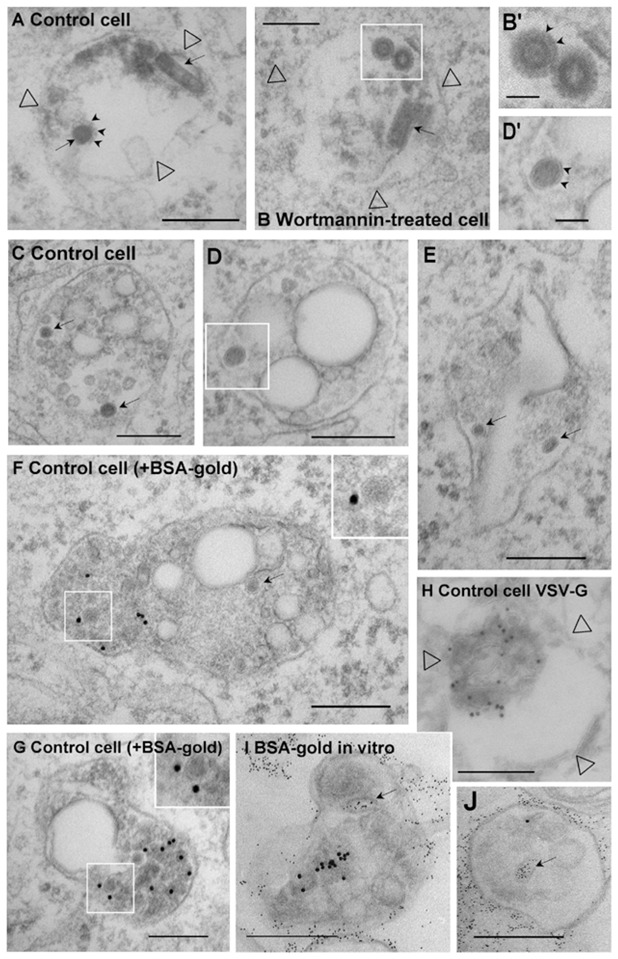
(A–H) After pre-binding to the surface (Fig 2D), VSV (100μg per 106 cells) was co-endocytosed with (F–G) or without (A-E and H) 10nm BSA-gold (OD = 1.7) for 10 min at 37°C, and then for 40 min without BSA-gold, in BHK cells treated (B) or not (A and C–H) with wortmannin. Cells were then processed for electron microscopy. (A–B) These panels show endosome (empty arrowheads point at the membrane) largely devoid of internal vesicles, presumably early endosomes, which contain both bullet-shaped virions and virions viewed in cross-section (arrows; small arrows point at G-protein spikes). (C–G) Electron-dense structures without a visible spike-delineated envelope, presumably capsids (arrows) are found within internal vesicles (see insets and the boxed area in D shown in D′; note clear membrane indicated by arrowheads in D′) of late endosomes containing gold particles in their lumen (F–G). (H) Experiment as in (A) and then the G-protein distribution was analyzed by immunogold labeling of cryo-sections using anti-VSV-G antibodies (empty arrowheads point at the membrane): note the characteristic electron-lucent space that is also observed in plastic embedded samples (C–G). (I–J) The G-protein was endocytosed for 45 min at 37°C into BHK cells 24 after labeling at the cell surface with a polyclonal antibody against G and then with 10nm proteinA-gold. Endosomal fractions were then prepared and incubated in vitro (as in Fig 8A) at 4°C with 4nm BSA-gold, and processed for electron microscopy. Note that BSA-gold labels apparently internal structures (arrows), which must have continuity with the limiting membrane (and are equivalent to cytoplasmic space) out of the plane of section. Bars: A–J: 0.2μm; B′-D′: 0.05μm).
