Abstract
The mechanisms by which infants and children process pain should be viewed within the context of a developing sensory nervous system. The study of the neurophysiological properties and connectivity of sensory neurons in the developing spinal cord dorsal horn of the intact postnatal rat has shed light on the way in which the newborn central nervous system analyzes cutaneous innocuous and noxious stimuli. The receptive field properties and evoked activity of newborn dorsal horn cells to single repetitive and persistent innocuous and noxious inputs are developmentally regulated and reflect the maturation of excitatory transmission within the spinal cord. These changes will have an important influence on pain processing in the postnatal period.
Increasing recognition of the importance of pain in infancy and childhood has focused attention on the basic neurobiology of developing pain pathways. After birth, many regions of the somatosensory nervous system undergo changes in connectivity, leading to transient functional stages before the adult pattern is achieved. Such changes are likely to determine pain and sensory processing at each developmental stage.
The aim here is to discuss some of the changing features of sensory connections underlying pain processing in the dorsal horn of the spinal cord over the course of postnatal development. The responses of newborn dorsal horn cells to single repetitive and persistent innocuous and noxious inputs are shown to change over the postnatal period. These are discussed in terms of the maturation of excitatory transmission within the spinal cord and how it may influence pain processing in the newborn.
Cutaneous Reflex Function in the Newborn.
Although cutaneous reflexes are not evidence of pain perception as such, they can provide information about the sensitivity and selectivity of the neonatal nervous system to nociceptive stimuli. A feature of the cutaneous flexion reflex in the newborn rat, kitten, and human is that it is exaggerated compared with the adult (1–4). Thresholds to mechanical skin stimulation are lower and the reflex muscle contractions more synchronized and long lasting (5, 6). This feature is particularly marked in the first postnatal week in rats, and then gradually changes until the third postnatal week when a rapid maturation takes place. Repeated low-intensity skin stimulation results in hyperexcitability or sensitization of the reflex with lowered thresholds and generalized movements of all limbs that becomes much less pronounced after 29- to 35-wk gestational age in the human and postnatal day 8 (P8) in the rat (4, 5). In addition, flexor reflex cutaneous receptive fields are larger (4) and less organized (6) than in the adult. Thresholds for withdrawal from heat stimuli are also lower in younger animals (7–11), and the response to formalin has a 10-fold higher sensitivity in neonatal rats compared with weanlings (12, 13). The specific C-fiber irritant, mustard oil, is less effective at producing a nociceptive response in the newborn; however, it gradually increases with postnatal age (14, 15).
Because the thresholds of cutaneous mechanosensitive primary afferents are generally the same in the adult and the neonatal rat (16), these postnatal changes in reflex sensitivity are likely to be caused by changes in central processing. The newborn spinal cord is clearly in a generally more excitable state than in the adult, and one possibility is that both low- and high-intensity stimuli can activate spinal pathways that are purely nociceptive in the adult, so neonatal A-fibers can evoke excitatory synaptic processes normally restricted to C-fiber input in adults. Studies of the changing sensory connectivity in developing dorsal horn neurons have provided some insight into this possibility.
Growth of Primary Afferent Terminals in the Newborn Spinal Cord.
Although large-diameter dorsal root afferent collaterals begin to grow into the dorsal grey at E15 (embryonic day 15) in the rat (refs. 17, 18; A. Jackman and M.F., unpublished observations), C-fibers grow into the spinal cord considerably later, at E19 onwards (19), and many chemical markers associated with C-fibers are not apparent in the spinal cord until the perinatal period. C-type afferent terminals within synaptic glomeruli are not observed at electron microscopy level until P5 (20).
The growth of both A- and C-fibers into the cord is somatotopically precise (19, 21, 22), but this is not true of the laminar organization. Although in the adult, Aβ afferents are restricted to laminae III and IV, in the neonate their terminals extend dorsally right up into laminae I and II to reach the surface of the grey matter (23, 24). This pattern is followed by a gradual withdrawal from the superficial laminae over the first three postnatal weeks (23). C-fibers, on the other hand, grow specifically to laminae I and II, and for a considerable postnatal period, these laminae are occupied by both A- and C-fiber terminals (23). During their occupation of superficial laminae, A-fiber terminals can be seen to form synaptic connections at electron microscopy level (25). Furthermore, during this period c-fos expression can be evoked in superficial dorsal horn cells in response to an innocuous or Aβ-strength skin stimulus (26), whereas in the adult, c-fos expression is normally induced only by noxious skin or Aδ- and C-fiber nerve stimulation. Fos induction is triggered only by innocuous stimulation in the adult under pathological conditions (27).
Postsynaptic Responses to Primary Afferent Stimulation in the Newborn Spinal Cord.
The importance of A-fiber afferent input in the newborn dorsal horn can be seen in an analysis of the extracellularly recorded spike activity evoked in individual cells in anesthetized rat pups (28). Low-intensity electrical skin stimulation (100 μA–3.5 mA, 50–200 μs) sufficient to recruit A-fibers evokes spike activity in both superficial and deep laminae at latencies that progressively decrease with age. At P3, the mean latency of the A-fiber-evoked response is 33.1 ± 2.78 ms (n = 22), compared with 19.1 ± 1.32 ms (n = 65) at P6, 13.5 ± 0.8 ms (n = 53) at P10, and 7.3 ± 0.3 ms (n = 35) at P21. Furthermore, the variation in the A-fiber latencies within the population of recorded cells decreases with age (28).
In contrast to responses to A-fiber input, no long-latency C-fiber-evoked (1–5 mA, 500 μs) spike responses are evoked in dorsal horn cells in vivo in the first postnatal week (28, 29). At P10, only 7 of 53 cells (13%) have a C-fiber input with a mean latency of 97.65 ± 4.44 ms (n = 7), and at P21 the value is 32% with a mean latency of 107.0 ± 10.12 ms (n = 10). These results do not, of course, provide information about subthreshold C-fiber-evoked responses at this time.
Convergence of Afferent Inputs in the Postnatal Dorsal Horn Cells.
Low-threshold inputs can also be seen to dominate the newborn dorsal horn when the responses to natural stimulation are examined. Background activity is generally absent when neonatal cells are initially isolated for recording, but strong responses can be evoked by mechanical stimulation of the skin of the receptive field. Some cells respond to both innocuous brushing and noxious pinching of the skin, but the convergence of input to dorsal horn cells changes over the postnatal period (Table 1). The responses recorded from cells in the younger animals are elicited mainly by low-threshold mechanoreceptors, and there are few cells with convergent input in the first week of life. This population gradually increases so that by P21 the percentage of neurones with convergent primary afferent input is similar to that seen in the adult.
Table 1.
Convergence of afferent input to dorsal horn cells at different postnatal ages
| Age | Cells
with different input
|
||
|---|---|---|---|
| Brush | Pinch | Brush and Pinch | |
| P3 (n = 22) | 20 (91%) | 1 (4.5%) | 1 (4.5%) |
| P6 (n = 65) | 54 (83%) | 7 (11%) | 4 (16%) |
| P10 (n = 53) | 22 (42%) | 12 (22%) | 19 (36%) |
| P21 (n = 35) | 10 (29%) | 5 (14%) | 20 (57%) |
Receptive Fields of Postnatal Dorsal Horn Cells.
The size of dorsal horn cell peripheral cutaneous receptive field decreases with age (30). We have studied this in more detail using those cells with mechanoreceptive fields on the plantar surface of the hindpaw. Receptive field areas were scanned into a computer, converted into pixels by using Adobe photoshop software (Adobe Systems, Mountain View, CA) and expressed as a percentage of the entire plantar surface of the hindpaw. At P3, the mean (±SE) peripheral receptive field occupies 50 ± 5.6% of the plantar hindpaw. This value drops to 36 ± 2.9% at P6, 20 ± 1.9% at P10, and 15% ± 1.6 at P21. The biggest change, therefore, occurs in the first postnatal week.
In the neonate, therefore, receptive fields are not only dominated by low-threshold inputs but are also larger and will therefore overlap more than in the adult, increasing the chance of activation by peripheral skin stimulation.
Activity-Dependent Changes in Postnatal Dorsal Horn. (i) Repetitive stimulation of receptive fields.
C-fiber-evoked activity is not observed in dorsal horn cells in the first postnatal week, and repetitive peripheral stimulation at C-fiber strength also has no observable effects on dorsal horn cell spike responses. From P10, repetitive C-fiber stimulation at three times the C-fiber threshold produces a classical “wind-up,” as reported in the adult dorsal horn (31–37) in 18% of cells. This percentage has increased to 40% of cells by P21.
In contrast to the lack of C-fiber influence, stimulation of cells at twice the A-fiber threshold at a frequency of 0.5 Hz through pin electrodes placed in the center of the peripheral receptive field on the hindlimb can produce considerable sensitization of the dorsal horn cells in the neonate (28). This sensitization takes the form of a buildup of background activity in the cells during repetitive stimulation that outlasts the stimulation period, thereby producing a prolonged after-discharge of up to 138 s. It is particularly apparent in younger animals, and at P6, 19 of 57 cells (33%) display background firing during, and a prolonged after-discharge of, 70.6 ± 18 s after repetitive A-fiber stimulation. At P10, 3 of 48 cells showed this type of sensitization (6%) with an after-discharge of 63 s, whereas at P21, it was not seen in any cells (n = 31) (28).
A-fiber-induced sensitization is not accompanied by an increase in the direct A-fiber-evoked spike discharge, but during the stimulation period, the sensitized units show a significant increase in activity outside of this short-latency evoked burst (28). The mean activity during the stimulation period, measured in the 40- to 2,000-ms period between stimuli, is 2.6 ± 0.16 spikes in P6 sensitizing cells, significantly greater (p < 0.0001) than the 0.4 ± 0.04 spikes in nonsensitizing cells. At P10, there is a similar pattern; the mean background activity for sensitizing cells was 15.7 ± 0.84 spikes, whereas that of nonsensitized cells was 1.3 ± 0.13 spikes, another significant difference (p < 0.0001).
(ii) Experimental inflammation in rat pups.
Carrageenan is reported to be a reliable agent in modeling inflammation in adults (38–44). After subcutaneous injection, edema develops rapidly, followed by hyperalgesia, which peaks at 3–4 hr and decreases to baseline by 24–72 hr (40–42). In some cases, the period of hyperalgesia can last 10–14 days (40). In view of the differences in sensory processing in the newborn compared with the adult dorsal horn, we examined the responses of newborn dorsal horn cells to a carrageenan inflammatory stimulus.
The allodynia or drop in mechanical threshold that follows carrageenan injection (11, 40) and hyperalgesia after mustard oil application (15) is clear, but smaller in amplitude, in neonates. Carrageenan-induced inflammation produces a parallel fall in von Frey thresholds at P3 and P10, whereas P21 animals show a significantly greater effect. At all ages, the effect increases with time, reaching a maximum at 4 hr postinjection, but is still maintained at 5 hr postinjection (11).
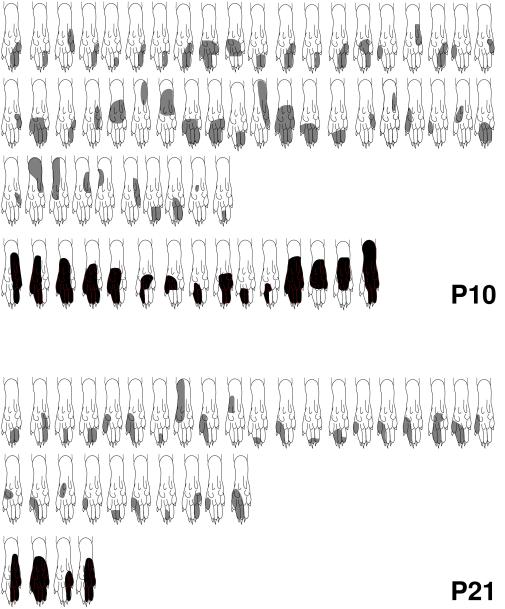
This finding agrees with the responses of dorsal horn cells at this time. After carrageenan injection, dorsal horn cell receptive fields recorded in anesthetized rat pups in vivo were measured and expressed as a percentage of the plantar foot area (Fig. 1). There was a significant increase in the size of peripheral receptive fields in animals in both the P10 and P21 age groups (P < 0.0001 in both cases). At P10, the size of the peripheral receptive fields increased 2.5-fold, and at P21 the increase was 3.4-fold. Mean size ±SEM (as a percentage of the plantar hindpaw area) of the peripheral receptive field in the inflamed group at P10 was 47.2 ± 6.4%, and that of the control was 19.1 ± 2.0%. At P21, the mean size of the receptive field in the inflamed group was 51.8 ± 12.2% and that of the control group was 14.9 ± 1.6%.
Figure 1.
Peripheral receptive fields: change with age and after inflammation. Representations of the peripheral receptive fields. Those in black represent cells receiving afferents from inflamed skin, whilst those in grey are the control. The mean receptive field size ±SEM (as a percentage of the total plantar foot area) is 50 ± 5.6% at P3, 36 ± 2.9% at P6, 20 ± 1.97% at P10, and 15 ± 1.62% at P21. For those with inflamed feet, the receptive field sizes are 47 ± 6.4% at P10 and 52 ± 12.2% at P21.
The receptive fields of adult dorsal horn neurons observed between 4 and 8.5 hr after injection of complete Freund’s adjuvant expanded to 2.4 times their original size (45). This increase of the receptive field may be responsible for hyperalgesia. Because the receptive fields are larger, there is a greater degree of overlap, and so a single stimulus would activate many more neurons than in the control state, a summative effect (46).
Effects of Primary Afferent Stimulation.
The magnitude of the evoked response (number of spikes) after electrical stimulation at twice A-fiber threshold directly to the nerve significantly increased after inflammation in P10 cells. Mean ± SEM evoked response for the control animals was 3.2 ± 0.25 spikes, and for the inflamed animals it was 7.6 ± 0.21 spikes. The Student’s t test gives a p < 0.0001 when comparing these two groups, suggesting that the difference is extremely significant.
In addition, the number of cells with C-fiber-evoked activity increased from 0/6 in the control group to 4/7 in the inflamed group. Even such small numbers suggest an important effect of the carrageenan-induced central excitation on “unmasking” C-fiber-evoked spike activity.
At P21, the mean magnitude of the evoked response for cells receiving an A afferent input was 6.8 ± 0.32 spikes in the control group and 6.6 ± 0.21 spikes in the inflamed group. The difference between these two groups is not significant (P = 0.59). For those cells responding to C afferent input, the magnitude of response was 4.9 ± 0.5 spikes for the control group and 10.1 ± 0.67 spikes for the inflamed group. These two results were significantly different, with a P < 0.0001.
A Possible Role for N-methyl-d-aspartate (NMDA) Receptors.
The results demonstrate important differences in the synaptic connectivity underlying sensory processing in the newborn spinal cord. The slow maturation of C-fiber afferent input appears to result in a predominance of A-fiber-evoked activity, such that processes that are exclusively activated by small-diameter nociceptive inputs in adults can be triggered by low-threshold large-diameter inputs in the first postnatal weeks.
There are likely to be several mechanisms underlying this transient state of A-fiber-induced excitation, but we would like to propose that one important one could be in the developmental regulation of NMDA receptors.
The neonatal spinal cord has a higher concentration of NMDA receptors in the grey matter than that observed in older animals (47). All laminae in the dorsal horn are uniformly labeled with NMDA-sensitive [3H]glutamate until day 10–12, when higher densities gradually appear in substantia gelatinosa so that by P30, binding is similar to that in the adult. Furthermore, the affinity of the receptors for NMDA and the NMDA-evoked calcium efflux in rat substantia gelatinosa is high in the first postnatal week and then declines (48). This maturation is delayed by neonatal capsaicin treatment, suggesting that C-fiber afferent activity regulates the postnatal maturation of NMDA receptors (48). There is also considerable rearrangement of the subunit composition of the NMDA channel complex during spinal cord development (49).
Acknowledgments
This study was supported by a European Community Biomedicine and Health grant (BMH4-CT95-0172). E.A.J. is a graduate student supported by the Medical Research Council of Great Britain and the Department of Anatomy, University College London.
ABBREVIATIONS
- P
postnatal day
- E
embryonic day
- NMDA
N-methyl-d-aspartate
References
- 1.Ekholm J. Acta Physiol Scand Suppl. 1967;297:1–130. [PubMed] [Google Scholar]
- 2.Issler H, Stephens J A. J Physiol (London) 1983;335:643–654. doi: 10.1113/jphysiol.1983.sp014556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald M, Gibson S. Neuroscience. 1984;13:933–944. doi: 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- 4.Andrews K, Fitzgerald M. Pain. 1994;56:95–101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald M, Shaw A, MacIntosh N. Dev Med Child Neurol. 1988;30:520–526. doi: 10.1111/j.1469-8749.1988.tb04779.x. [DOI] [PubMed] [Google Scholar]
- 6.Marsh, D., Dickenson, A. H., Hatch, D. & Fitzgerald, M. (1999) Pain, in press.
- 7.Holmberg H, Schouenborg J. J Physiol (London) 1996;493:239–252. doi: 10.1113/jphysiol.1996.sp021379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewin G R, Ritter A M, Mendell L M. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcon M, Guendellman D, Stolberg A, Frenk H, Urca G. Pain. 1996;67:203–208. doi: 10.1016/0304-3959(96)03070-9. [DOI] [PubMed] [Google Scholar]
- 10.Hu D, Hu R, Berde C B. Anesthesiology. 1997;86:957–965. doi: 10.1097/00000542-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Marsh, D., Dickenson, A. H., Hatch, D. & Fitzgerald, M. (1999) Pain, in press.
- 12.Guy E R, Abbott F V. Pain. 1992;51:81–90. doi: 10.1016/0304-3959(92)90012-Z. [DOI] [PubMed] [Google Scholar]
- 13.Teng C J, Abbott F V. Pain. 1998;76:337–347. doi: 10.1016/S0304-3959(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald M, Gibson S. Neuroscience. 1984;13:933–944. doi: 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M C, Gebhart G F. Pain. 1998;77:305–313. doi: 10.1016/S0304-3959(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald M. J Physiol (London) 1987;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C L. J Comp Neurol. 1983;220:29–43. doi: 10.1002/cne.902200105. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald M, Reynolds M L, Benowitz L I. Neuroscience. 1991;41:187–199. doi: 10.1016/0306-4522(91)90209-7. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald M. J Comp Neurol. 1987;261:98–104. doi: 10.1002/cne.902610108. [DOI] [PubMed] [Google Scholar]
- 20.Pignatelli D, Ribeiro da Silva A, Coimbra A. Brain Res. 1989;491:33–44. doi: 10.1016/0006-8993(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald M, Swett J. Neurosci Lett. 1983;43:149–154. doi: 10.1016/0304-3940(83)90179-9. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki S, Snider W D. J Comp Neurol. 1997;380:215–229. [PubMed] [Google Scholar]
- 23.Fitzgerald M, Butcher T, Shortland P. J Comp Neurol. 1994;348:225–233. doi: 10.1002/cne.903480205. [DOI] [PubMed] [Google Scholar]
- 24.Mirnics K, Koerber H R. J Comp Neurol. 1995;355:601–614. doi: 10.1002/cne.903550409. [DOI] [PubMed] [Google Scholar]
- 25.Coggeshall R E, Jennings E A, Fitzgerald M. Brain Res Dev Brain Res. 1996;92:81–90. doi: 10.1016/0165-3806(95)00207-3. [DOI] [PubMed] [Google Scholar]
- 26.Jennings E, Fitzgerald M. Pain. 1996;68:301–306. doi: 10.1016/s0304-3959(96)03194-6. [DOI] [PubMed] [Google Scholar]
- 27.Ma Q P, Woolf C J. Pain. 1996;67:307–316. doi: 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- 28.Jennings E, Fitzgerald M. J Physiol (London) 1998;509:859–868. doi: 10.1111/j.1469-7793.1998.859bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald M. Neurosci Lett. 1988;86:161–166. doi: 10.1016/0304-3940(88)90564-2. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald M. J Physiol (London) 1985;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendell L M, Wall P D. Nature (London) 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- 32.Mendell L M. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- 33.Woolf C J, Thompson S W, King A E. J Physiol (Paris) 1988;83:255–266. [PubMed] [Google Scholar]
- 34.Davies S N, Martin D, Millar J D, Aram J A, Church J, Lodge D. Eur J Pharmacol. 1988;145:141–151. doi: 10.1016/0014-2999(88)90225-7. [DOI] [PubMed] [Google Scholar]
- 35.Dickenson A H, Sullivan A F. Brain Res. 1990;506:31–39. doi: 10.1016/0006-8993(90)91195-m. [DOI] [PubMed] [Google Scholar]
- 36.Thompson S W, Woolf C J, Sivilotti L G. J Neurophysiol. 1993;69:2116–2128. doi: 10.1152/jn.1993.69.6.2116. [DOI] [PubMed] [Google Scholar]
- 37.Woolf C J, Thompson S W. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 38.Xu X J, Elfvin A, Wiesenfeld Hallin Z. Neurosci Lett. 1995;196:116–118. doi: 10.1016/0304-3940(95)11837-m. [DOI] [PubMed] [Google Scholar]
- 39.Winter C A, Risley E A, Nuss G W. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 40.Kayser V, Guilbaud G. Pain. 1987;28:99–107. doi: 10.1016/0304-3959(87)91064-5. [DOI] [PubMed] [Google Scholar]
- 41.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 42.Hylden J L, Thomas D A, Iadarola M J, Nahin R L, Dubner R. Eur J Pharmacol. 1991;194:135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- 43.Stanfa L C, Sullivan A F, Dickenson A H. Pain. 1992;50:345–354. doi: 10.1016/0304-3959(92)90040-I. [DOI] [PubMed] [Google Scholar]
- 44.Stanfa L C, Misra C, Dickenson A H. Brain Res. 1996;737:92–98. doi: 10.1016/0006-8993(96)00629-4. [DOI] [PubMed] [Google Scholar]
- 45.Hylden J L, Nahin R L, Traub R J, Dubner R. Pain. 1989;37:229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- 46.Dubner R, Ruda M A. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez D L, Fuchs J L, Droge M H. Neurosci Lett. 1993;151:134–137. doi: 10.1016/0304-3940(93)90004-5. [DOI] [PubMed] [Google Scholar]
- 48.Hori Y, Kanda K. Dev Brain Res. 1994;80:141–148. doi: 10.1016/0165-3806(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe M, Mishina M, Inoue Y. J Comp Neurol. 1994;345:314–319. doi: 10.1002/cne.903450212. [DOI] [PubMed] [Google Scholar]