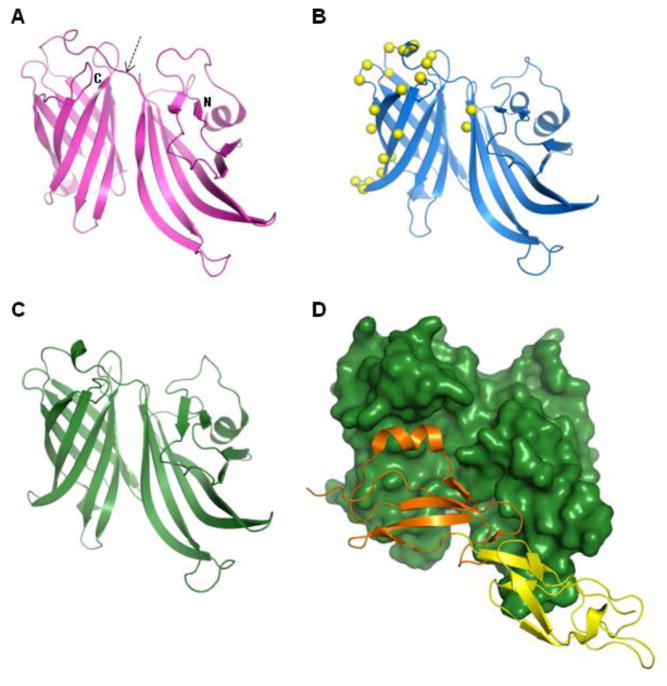
Figure 2. Structures of fHbp.
The currently known structures of the factor H binding protein (fHbp) from N. meningitidis serogroup B all share the same overall fold: two beta-barrel domains (one domain N-terminal and the other C-terminal, as indicated) connected by a short linker (arrowed), evident in panels A-C.(A) The NMR solution structure of a variant 1 fHbp from strain MC58 (PDB entry 2KC0) and (B) the crystal structure of an engineered form of fHbp variant 1 (strain MC58) containing 22 point mutations (PDB entry 2Y7S); the crystal structure of fHbp variant 1 has also been determined in complex with domains 6 and 7 of human Factor H (PDB entry 2W80): panel (C) shows the fHbp alone, panel (D) shows the same orientation of fHbp as a surface representation (dark green) with bound Factor H domains 6 (orange) and 7(yellow) shown as ribbons.