The clinical evidence supporting proteasome inhibitor combination therapy for B-cell non-Hodgkin's lymphoma is reviewed.
Keywords: Lymphoma, Non-Hodgkin's, Bortezomib, Carfilzomib, Marizomib, ONX-0912
Abstract
Although patients with B-cell non-Hodgkin's lymphoma (NHL) usually respond to initial conventional chemotherapy, they often relapse and mortality has continued to increase over the last three decades in spite of salvage therapy or high dose therapy and stem cell transplantation. Outcomes vary by subtype, but there continues to be a need for novel options that can help overcome chemotherapy resistance, offer new options as consolidation or maintenance therapy postinduction, and offer potentially less toxic combinations, especially in the elderly population. The bulk of these emerging novel agents for cancer treatment target important biological cellular processes. Bortezomib is the first in the class of proteasome inhibitors (PIs), which target the critical process of intracellular protein degradation or recycling and editing through the proteasome. Bortezomib is approved for the treatment of relapsed or refractory mantle cell lymphoma. The mechanisms of proteasome inhibition are very complex by nature (because they affect many pathways) and not fully understood. However, mechanisms of action shared by bortezomib and investigational PIs such as carfilzomib, marizomib, ONX-0912, and MLN9708 are distinct from those of other NHL treatments, making them attractive options for combination therapy. Preclinical evidence suggests that the PIs have additive and/or synergistic activity with a large number of agents both in vitro and in vivo, from cytotoxics to new biologicals, supporting a growing number of combination studies currently underway in NHL patients, as reviewed in this article. The results of these studies will help our understanding about how to best integrate proteasome inhibition in the management of NHL and continue to improve patient outcomes.
Overview
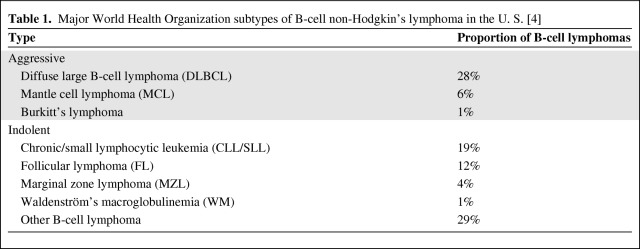
In the U.S., >70,000 patients are diagnosed with non-Hodgkin's lymphoma (NHL) annually [1], and one in three patients dies within 5 years of diagnosis [2]. Of note, the incidence of NHL has increased dramatically (>80% over the last three decades). The broad spectrum of malignancies comprising NHL can be classified according to cell of origin (B, T, or natural killer cell), as well as mature or immature phenotype, as seen in the World Health Organization classification (Table 1) [3, 4]. The distribution of the different subtypes of NHL varies geographically worldwide.
Table 1.
Major World Health Organization subtypes of B-cell non-Hodgkin's lymphoma in the U. S. [4]
Response rates to conventional chemotherapy are typically >50%, and many patients achieve a complete response (CR). Even so, most patients eventually relapse [5], and there is no generally accepted therapeutic approach for relapsed or refractory lymphoma [6] outside of Hodgkin's lymphoma and diffuse large B-cell lymphoma (DLBCL) after first relapse. The addition of rituximab to chemotherapy has clearly improved the objective response rate (ORR) and CR rate and has been the main improvement in the treatment of B-cell NHL during the last three decades. Though it varies with B-cell NHL subtype, a significant number of patients relapse with no greatly defined standard therapies. For DLBCL, the combination of high-dose chemotherapy and stem cell transplantation is effective for relapsed NHL [7] but is clearly worse in patients with early relapse or primary failures. Patients with indolent NHL relapse even more frequently, with a subsequent shorter response to chemotherapy and often transformation over time. For mantle cell lymphoma (MCL), dose-intensive approaches have clearly resulted in significantly longer progession-free survival (PFS) intervals, although such approaches are not applicable routinely because the median age at diagnosis is in the mid- to late 60s. Patients with relapsed or refractory MCL are often resistant to chemotherapy and have a poor outcome with conventional therapies or even high-dose therapy with stem cell transplantation.
The need for additional treatment options for NHL has led to the development of novel therapies. The reversible proteasome inhibitor (PI) bortezomib (Velcade®; Millennium Pharmaceuticals, Inc., Cambridge, MA and Johnson & Johnson Pharmaceutical Research & Development, LLC, Titusville, NJ) is an orphan drug approved in the United States and European Union for the treatment of multiple myeloma, and in the United States for the treatment of MCL after one prior therapy.
This review summarizes the clinical evidence supporting PI combination therapy for B-cell NHL. Bortezomib is the only PI currently approved for widespread clinical use and thus is the focus of this review; however, where data are available, studies of other PIs for NHL are also discussed.
Biologic Basis of PI Combination Therapy
Mechanisms of Action
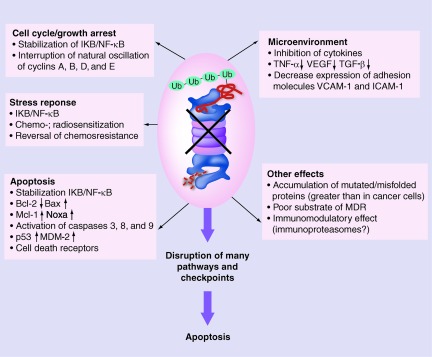
The proteasome is an intracellular, multiunit protease complex responsible for protein modification and degradation. Bortezomib binds to the β-subunits of the core of the proteasome, leading to disruption of intracellular protein degradation/recycling, and induces apoptosis (Fig. 1) [8]. A major mechanism of action for bortezomib is inactivation of nuclear factor (NF)-κB, via stabilization of the NF-κB inhibitor IκB [9–14], which contributes to apoptosis, cell cycle or growth arrest, and stress response. Bortezomib also enhances apoptosis by promoting the release of cytochrome C [15], stimulating generation of reactive oxygen species [16], upregulating the tumor suppressor protein p53 and the two inhibitors of cyclin-dependent kinase (CDK), p21 and p27 [11, 13, 15], and increasing expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and TRAIL receptors [17]. Bortezomib may also have numerous effects on the B-cell lymphoma 2 (Bcl-2) family of proteins, including activation of proapoptotic Bcl-2 proteins [10, 15, 16, 18] and inhibition of antiapoptotic Bcl-2 proteins [9, 16, 18]. Proteasome inhibition with bortezomib also contributes to cell cycle or growth arrest by interrupting oscillation of cyclins A, B, D, and E, but its cytotoxic activity in NHL may be independent of cyclin D1 levels [19]. In addition to the effects of NF-κB, the stress response can be enhanced through the effects of proteasome inhibition on chemosensitization [20] and radiosensitization, particularly in cell lines that are dependent on NF-κB [21, 22]. Proteasome inhibition may also influence the microenvironment by inhibiting cytokines, growth factors, and adhesion molecules [23]. Additional mechanisms of action of proteasome inhibition are still being elucidated.
Figure 1.
Bortezomib-mediated proteasome inhibition affects multiple signaling pathways.
Abbreviations: ICAM-1, intercellular adhesion molecule 1; IKB, NF-κB inhibitor; MDM-2, murine double minute 2; MDR, multidrug resistance; NF-κB, nuclear factor κB; TNF-α, tumor necrosis factor α; TGF-β, transforming growth factor β; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
Reproduced from Suh KS, Goy A. Bortezomib in mantle cell lymphoma. Future Oncol 2008;4:149–168, with permission from Future Medicine Ltd.
Other PIs are in development—but have not been approved for the treatment of NHL—such as the irreversible PIs carfilzomib [24] and marizomib (salinosporamide A, NPI-0052) [25] and the oral PIs ONX-0912 [26] and MLN9708 [27]. Bortezomib targets both the β5 subunit of the proteasome, also known as the chymotrypsin-like (CT-L) active site, and the β1 caspase-like sites, with less affinity for the β2 trypsin-like sites [28]. Carfilzomib has mechanisms of action that are similar to those of bortezomib but, unlike bortezomib, carfilzomib binds proteasomes irreversibly and is selective for the CT-L active site [29]. Marizomib also binds proteasomes irreversibly [30], leading to activation of caspase-8 [31, 32] and potentiation of apoptosis [23], but it inhibits all three sites for up to 7 days and it may be a more potent inhibitor of NF-κB than bortezomib [28, 33]. However, it remains to be determined whether or not differences in binding kinetics influence clinical efficacy and safety [34]. ONX-0912 (previously PR-047) has a chemical structure similar to that of carfilzomib and is also selective for the CT-L active site [35] but is orally available. MLN9708 is a boron-containing PI that is also orally available and has better tissue distribution than bortezomib [27].
Rationale for PI Combination Therapy
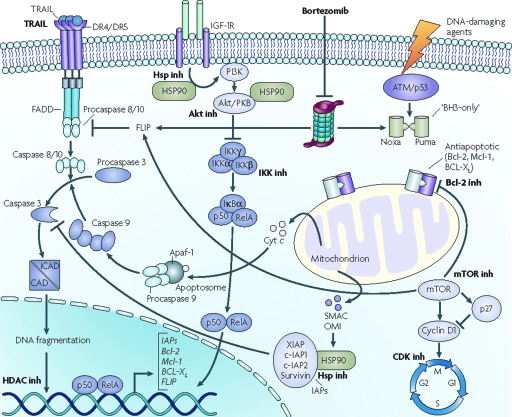
To maximize antitumor activity, NHL is commonly treated with combination chemotherapy regimens, such as cyclophosphamide, vincristine, and prednisone (CVP), cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), and cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide (EPOCH) [36, 37]. Because they have a distinct mechanisms of action and do not share mechanisms of chemoresistance with conventional chemotherapy or biologicals (Fig. 2), PIs are a rational addition to these agents.
Figure 2.
Schematic diagram of programmed cell death and altered pathways in mantle cell lymphoma.
Abbreviations: Bcl-2, B-cell lymphoma 2; CAD, caspase-activated DNase; CDK, cyclin-dependent kinase; Cyt, cytochrome; DR, death receptor; FADD, Fas-associated protein with death domain; FLIP, FLICE-inhibitory protein; HDAC, histone deacetylase; HSP90, heat shock protein 90; IAP, inhibitor of apoptosis protein; iCAD, inhibitor of caspase-activated DNase; IGF-1R, insulin-like growth factor 1 receptor; IKK, IκB kinase; inh, inhibitor; Mcl-1, myeloid leukemia cell differentiation protein 1; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B; SMAC, second mitochondria-derived activator of caspase; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; XIAP, X-linked inhibitor of apoptosis protein.
From Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: Perspectives for new targeted therapeutics. Nat Rev Cancer 2007;7:750–762 [163]. Reprinted by permission from Macmillan Publishers Ltd, © 2007.
In preclinical studies of NHL, apoptosis was induced synergistically when a PI was combined with established chemotherapeutic agents [38, 39] or immunotherapy (rituximab) [40–42]. Synergy in B-cell NHL was also reported in preclinical studies that combined a PI with several classes of investigational agents, including Bcl-2 inhibitors [20, 43–51], CDK inhibitors (e.g., flavopiridol) [39, 52], mammalian target of rapamycin inhibitors (e.g., everolimus) [53, 54], histone deacetylase inhibitors (e.g., suberoylanilide hydroxamic acid, romidepsin, belinostat, panobinostat, cyproheptadine) [55–64], other PIs [65, 66], protein kinase B (Akt) inhibitors [67], protein kinase C inhibitors [68], an inhibitor of Janus kinase/signal transducer and activation of transcription pathways [69], heat shock protein inhibitors [70], immunomodulation with interferon-γ [71], radioimmunotherapy [72], and TRAIL [73–75].
MCL
Background
MCL is characterized not only by the reciprocal translocation t(11;14)(q13;q32), which typically results in overexpression of cyclin D1, a key regulator of the cell cycle, but also by a clear genetic heterogeneity among patients, which likely explains the spectrum of disease seen in the clinic (e.g., indolent versus aggressive course).
There is no consensus on the optimal regimen for the treatment of MCL, but patients commonly receive rituximab plus fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (R–hyper-CVAD) [76] or induction chemotherapy followed by autologous stem cell transplantation in the frontline setting [77]. Dose-intensive approaches have clearly resulted in longer PFS intervals, >5 years, which is much better than the 18–22 months seen with rituximab plus CHOP (R-CHOP); however, patients still relapse and the impact on overall survival is debated. Furthermore, given that the median age at diagnosis is in the mid- to late 60s, non-intensive regimens and non-chemotherapy-based regimens are needed.
Although MCL is a largely incurable, aggressive disease with a poor overall prognosis, initial responses may exceed 50%, but most patients relapse and the median survival duration is approximately 3–4 years. The addition of rituximab to high-dose chemotherapy with [78] or without [76] stem cell transplantation lead to longer PFS times in untreated MCL patients. Thus, rituximab has become a standard component of MCL treatment regimens such as R-CHOP [79], R-bendamustine [80, 81], and infusional chemotherapy with R-EPOCH (R-CHOP plus etoposide) [82]. Consolidation with rituximab or radioimmunotherapy may also result in a longer response duration [83, 84]. Nonetheless, additional treatment options are needed to improve outcomes.
PI Monotherapy for MCL
Single-agent bortezomib was approved for the treatment of relapsed or refractory MCL on the basis of clinical evidence from several studies. The first evidence of clinical activity of bortezomib for NHL came from a phase I study of patients with refractory hematologic malignancies [85]. Two of 10 patients (20%) with NHL (1 of whom had MCL) had a partial response (PR) to bortezomib, 1.38 mg/m2 twice weekly for 4 weeks in 6-week cycles.
Several subsequent phase I/II trials of single-agent bortezomib for NHL reported responses (comprising all cases of a CR, unconfirmed CR [CRu], or PR) in up to 50% of patients, including a CR or CRu in up to 21% of patients [86–91]. In the pivotal phase II PINNACLE trial [92], 155 patients with relapsed or refractory MCL received single-agent bortezomib, 1.3 mg/m2 on days 1, 4, 8, and 11 of 21-day cycles, which is the standard dose and schedule for multiple myeloma. The response rate was 33% (CR and CRu rate, 8%), and after an extended follow-up of a median of 26.4 months, the median duration of response was not reached among patients achieving a CR or CRu and was 9.2 months among all responders [93]. The median overall survival times were 23.5 months in all patients and 35.4 months in responding patients, with an estimated 61.1-month survival duration from diagnosis. Of note, responses were seen even in patients who had failed prior high-dose or dose-intensive therapy or had bulky disease. In another phase II study of 40 heavily pretreated patients with MCL who received bortezomib, 1.5 mg/m2 on the standard schedule [94], the ORR was 45% (CR and CRu rate, 15%). The most common nonhematologic grade ≥3 adverse events (AEs) in these studies were fatigue, infection, neuropathy, and hyponatremia.
Analyses of biochemical markers in patients with MCL have confirmed that elevated NF-κB predicts better outcomes with bortezomib treatment, whereas bortezomib is not able to overcome the poor risk features of MCL associated with increased cellular proliferation as evidenced by elevated Ki-67 levels [95] or in patients with the blastoid morphologic variant.
A few studies have also reported the activity of investigational PIs as single-agent treatment for MCL. In a phase I study in 37 patients with multiple myeloma or relapsed NHL, carfilzomib (1.2–27 mg/m2) was given on days 1, 2, 8, 9, 15, and 16 of 28-day cycles; only three patients received the minimum effective dose of 15 mg/m2 or more, and none of those patients responded [96]. Dose-limiting toxicities included a hypoxic event and grade 4 thrombocytopenia. Another phase I study treated 29 patients with multiple myeloma or relapsed NHL with carfilzomib (1.2–20 mg/m2) for five consecutive days in 14-day cycles [97]. The minimum effective dose was 11 mg/m2, above which one of seven patients (14%) with NHL responded. Grade 3 (25%) or grade 4 (8%) AEs were primarily hematologic.
Marizomib (0.0125–0.55 mg/m2) once weekly for 3 weeks in 4-week cycles was evaluated in a phase I study of 30 patients with solid tumors and five patients with NHL; however, the maximum-tolerated dose was not reached and there were no responses in NHL patients [98].
PI Combination Therapy for MCL
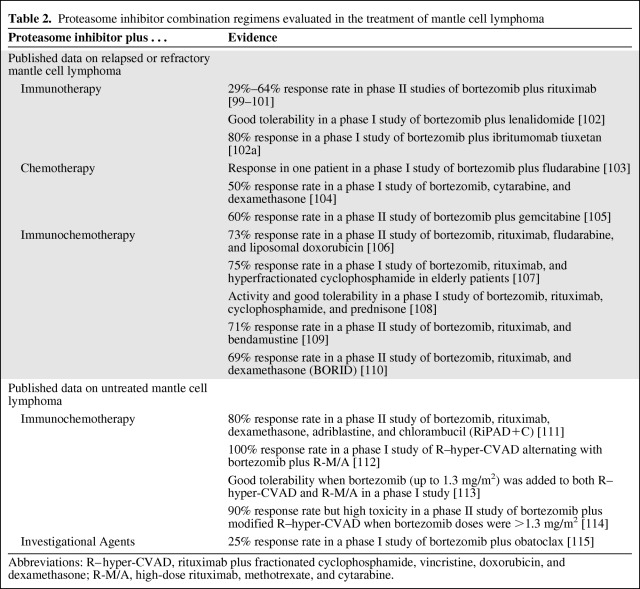
Numerous clinical studies have evaluated combinations of bortezomib with immunotherapy, chemotherapy, and immunochemotherapy in the treatment of MCL (Table 2).
Table 2.
Proteasome inhibitor combination regimens evaluated in the treatment of mantle cell lymphoma
Abbreviations: R–hyper-CVAD, rituximab plus fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; R-M/A, high-dose rituximab, methotrexate, and cytarabine.
Bortezomib and rituximab are both approved for the treatment of MCL and they have different safety profiles, supporting their combination in MCL treatment. When bortezomib was combined with rituximab in phase II studies [99–101], 29%–64% of patients with relapsed or refractory MCL responded to the combination; neurologic, gastrointestinal, and hematologic toxicities were common but generally reversible. Interim results of another phase II study suggested that the combination of bortezomib with the immunomodulator lenalidomide may also be active and may be well tolerated in the treatment of patients with relapsed or refractory MCL [102]. A phase I study that combined bortezomib with ibritumomab tiuxetan reported responses in 80% of patients with MCL [102a].
Bortezomib may potentiate fludarabine activity by inhibiting DNA repair and inducing apoptosis in Bcl-2–overexpressing cells. A phase I study of this combination [103] reported a PR in one patient with MCL, but two others with MCL discontinued treatment because of cytopenias. In another phase I study of heavily pretreated patients with MCL who received bortezomib, cytarabine, and dexamethasone [104], the ORR was 50%, but all patients had grade 3–4 hematologic toxicity. Among 26 patients with relapsed MCL who received bortezomib plus gemcitabine in a phase II study, the response rate was 60% and the median PFS interval was 11.4 months [105].
Several studies have reported activity with the combination of bortezomib with both immunotherapy (rituximab) and chemotherapy for relapsed or refractory MCL. Bortezomib, rituximab, fludarabine, and liposomal doxorubicin was found to result in an ORR of 73% in patients with relapsed or refractory MCL [106]. The combination of bortezomib, rituximab, and hyperfractionated cyclophosphamide was well tolerated in a phase I study of elderly patients (72–84 years of age) and resulted in an ORR of 75% [107]. Adding prednisone to this combination (i.e., substituting bortezomib for vincristine in the R-CVP regimen) was also active and well tolerated in another phase I study [108]. The combination of rituximab, bortezomib, and bendamustine in six 28-day cycles was evaluated in a phase II study of 30 patients, including seven with MCL [109]. Most patients (83%) had objective responses and the 2-year PFS rate was 47%. Within MCL patients, the overall response rate was 71%. The combination of bortezomib, rituximab, and dexamethasone (BORID) resulted in a 69% response rate in a phase II study of 16 patients with heavily pretreated MCL [110]. In another phase II study, adding doxorubicin and chlorambucil to BORID resulted in an 80% response rate [111].
Bortezomib has also been evaluated in combination with intense immunochemotherapy for untreated MCL. A phase I study that alternated hyper-CVAD with bortezomib plus high-dose rituximab, methotrexate, and cytarabine (R-M/A) reported objective responses in all 15 patients [112]. Adding bortezomib to both the R–hyper-CVAD and R-M/A cycles was well tolerated when the dose of bortezomib that was added to R-M/A was increased from 0.7 mg/m2 to 1.0–1.3 mg/m2 [113]. Toxicities with the addition of bortezomib were similar to the toxicities that would be expected without bortezomib. A phase II study that added bortezomib and rituximab to modified hyper-CVAD resulted in a response rate of 90% [114]. In that study, sensory neuropathy required the doses of bortezomib and vincristine to be decreased from 1.5 mg/m2 and 2 mg, respectively, to 1.3 mg/m2 and 2 mg, respectively, and then to 1.3 mg/m2 and 1 mg, respectively, in subsequent cohorts to improve tolerability [114]. On the basis of these studies, the standard bortezomib dose of 1.3 mg/m2 appears to be appropriate for further evaluation in combination with rituximab and hyper-CVAD regimens. Several studies are under way to evaluate the activity and tolerability of bortezomib with these and other widely used treatments for untreated MCL. Other studies are evaluating the use of bortezomib maintenance or consolidation therapy after remission with bortezomib plus R-CHOP, but data from those studies are not yet available.
Obatoclax is a small-molecule inhibitor of all members of the Bcl-2 family of prosurvival proteins. Bortezomib increases levels of Mcl-1, which is a Bcl-2 family member, and the combination of bortezomib and obatoclax was synergistic in a preclinical study [45]. A dose-finding phase I study of bortezomib plus obatoclax reported an ORR of 33% in patients with relapsed or refractory MCL [115]. Many other clinical studies are under way to evaluate the addition of bortezomib or an investigational PI to conventional chemotherapy or investigational agents for MCL (supplemental online Table A). Results of those studies are eagerly awaited.
Follicular Lymphoma
Background
Follicular lymphoma (FL) is a common type of indolent NHL that is associated with translocation of chromosomes 14 and 18, resulting in constitutive activation of the BCL2 oncogene and inhibition of apoptosis [116]. Because FL is also associated with constitutive activation of NF-κB [117], bortezomib is a rational treatment choice on the basis of its inhibition of NF-κB. The indolent types of B-cell NHL, including FL, are not considered curable. The addition of immunotherapy to conventional chemotherapy has improved overall survival significantly in FL [116]. The addition of maintenance therapy has continued to extend PFS; nevertheless, a large number of patients still relapse, illustrating the need for novel therapies in this setting [83].
Single-Agent PI Therapy for FL
Single-agent PI treatment has only modest activity for FL. In a phase II study, the standard bortezomib dose (1.3 mg/m2 twice weekly) resulted in objective responses in only six of 36 patients (17%) with relapsed or refractory FL [118]. A randomized, phase II study that compared two dosing schedules of single-agent bortezomib for FL reported response rates of 30% (15 of 50 patients) with twice-weekly dosing and 22% (eight of 37 patients) with once-weekly dosing [119]. Two other phase II studies demonstrated higher response rates of 55%–77% following twice-weekly bortezomib, 1.3–1.5 mg/m2, but those studies each included only nine to 11 evaluable patients [87, 99], and dose-limiting neurologic toxicity was seen at the higher dose [99]. It may be necessary to continue treatment for FL longer before a response to PI therapy is seen. A phase II study in patients with various types of relapsed or refractory NHL reported that 50% of patients with FL responded to single-agent bortezomib after a median of 11–12 weeks, compared with a median time to treatment response of only 4 weeks for MCL [120].
PI Combination Therapy for FL
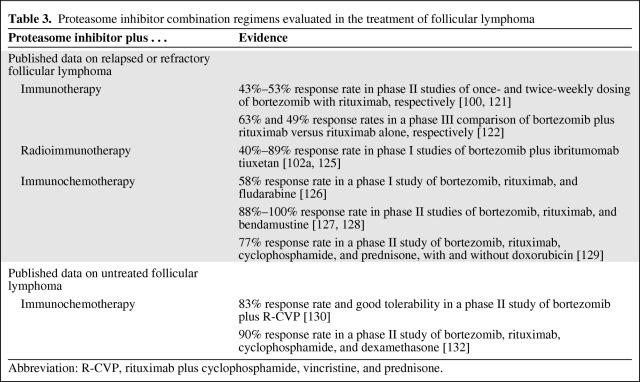
Table 3 summarizes published results of PI combinations for FL. Adding bortezomib to rituximab may improve treatment outcomes in FL patients. Among patients with relapsed FL, a phase II study of once-weekly or twice-weekly bortezomib dosing in combination with rituximab reported response rates of 43% and 49%, respectively [121]. In another randomized, phase II study of once-weekly or twice-weekly bortezomib combined with rituximab, the response rate was 53% among patients with recurrent FL, but neurologic, gastrointestinal, and hematologic toxicities were common [100]. Data from a randomized, phase III study of 676 patients with relapsed FL suggested that the combination of bortezomib and rituximab was significantly more effective than rituximab alone [122] in terms of the response rate (63% versus 49%; p < .001) and median PFS interval (389 days versus 334 days; p = .039), and the median overall survival time was not reached in either group after a median follow-up of 33.9 months. Although the absolute 1.8-month longer PFS duration overall did not achieve the prespecified goal of a 33% improvement, there were statistically significant improvements in patients with high-risk FL by Follicular Lymphoma International Prognostic Index score (11.4 months versus 7.9 months; p = .013) or by tumor burden (11.3 months versus 8.4 months; p = .019). Further analysis of the data identified individual biomarkers and pairs of biomarkers that may aid identification of subgroups deriving maximal benefit from the addition of bortezomib to rituximab therapy [123]. An ongoing, phase II study is comparing the activity of bortezomib and fludarabine with rituximab and fludarabine among patients with FL who have received previous rituximab treatment [124].
Table 3.
Proteasome inhibitor combination regimens evaluated in the treatment of follicular lymphoma
Abbreviation: R-CVP, rituximab plus cyclophosphamide, vincristine, and prednisone.
An emerging treatment strategy for NHL involves administration of radioimmunotherapy, and the addition of a PI may improve the response to these treatments. Ibritumomab tiuxetan combines a monoclonal antibody with a linker-chelator that has high affinity for radioactive yttrium-90 or indium-111. Like rituximab, ibritumomab binds to the CD20 antigen on the surface of normal and malignant B cells, allowing radiation from the attached isotope to kill the B cells, and ibritumomab itself may also trigger cell death. Response rates of 40%–89% were reported in phase II studies that combined bortezomib and ibritumomab tiuxetan [102a, 125].
Several studies have reported activity for the combination of bortezomib with immunochemotherapy. A response rate of 58% (seven of 12 patients) was reported among patients with relapsed or refractory FL who received bortezomib, rituximab, and fludarabine in a phase I dose-finding study [126]. Bortezomib, rituximab, and bendamustine was associated with excellent response rates of 88%–100% in phase II studies of 49 patients and 11 patients, respectively, with relapsed or refractory FL [127, 128]. In a phase II, nonrandomized, two-arm study of 55 patients with relapsed or refractory FL, bortezomib, rituximab, cyclophosphamide, and prednisone led to a 78% response rate, and the addition of doxorubicin resulted in a 75% response rate [129].
Less is known about the activity of PI combination therapy in patients with previously untreated FL. In this patient population, the addition of bortezomib to R-CVP was effective and very well tolerated in a phase II study [130]. That study and one of the studies of relapsed or refractory FL [128], which were published concurrently, did not meet their primary endpoint of higher CR rates than in historical controls. An accompanying editorial examined the limitations of these studies, questioned whether or not selected groups of patients should be evaluated for benefits from bortezomib-containing combination immunochemotherapy for FL and highlighted the importance of randomized data in clinical decision making [131].
In another phase II study, the addition of bortezomib to rituximab, cyclophosphamide, and dexamethasone also had an acceptable safety profile in elderly patients with untreated, predominantly indolent subtypes of NHL, and the combination was associated with a response rate of 90% [132].
Consolidation with PI therapy may also be beneficial for patients with indolent NHL, but this has not been studied extensively. Consolidation therapy for FL with bortezomib, 1.6 mg/m2 once weekly for 3 weeks in 4-week cycles, has been reported after combination treatment with bortezomib and ibritumomab tiuxetan [125].
DLBCL
Background
DLBCL is the most common subtype of NHL (almost one third of patients [4]) and is typically treated with rituximab plus anthracycline-based chemotherapy (R-CHOP or R-EPOCH). The addition of rituximab has dramatically improved outcomes of DLBCL, though patients who fail R-CHOP chemotherapy usually do poorly with salvage chemotherapy, especially if they relapse within 12 months. Eighty percent of relapses in DLBCL after R-CHOP occur within the first 2 years, representing therefore a difficult challenge in practice. Multiple prognostic factors have been identified, including IPI, proliferation index (high Ki-67), and molecular subtype (activated B-cell origin [ABC] and germinal center B cell-like [GCB]) [133, 134]. Though the outcomes of both GCB and ABC subtypes have been improved by chemoimmunotherapy [135], the ABC subtype continues to have a significantly worse outcome than GCB [136, 137]. A better understanding of the biology of DLBCL has shown that NF-κB and activation of the B-cell receptor pathway are critical in the ABC subtype. Thus, investigators are looking at strategies to improve outcomes of DLBCL, especially by combining novel therapies with R-CHOP, in the hope of decreasing the incidence of early failures.
Single-Agent PI Therapy for DLBCL
Preclinical data and the understanding of the biology of DLBCL provided a rationale for proteasome inhibition in DLBCL [138]. Despite its inhibition of the NF-κB pathway, single-agent PI therapy appears to have little clinical utility in this patient population. In a phase I/II study of patients with relapsed or refractory DLBCL, bortezomib monotherapy had minimal activity (1 PR) [139]. To our knowledge, single-agent studies of carfilzomib, marizomib, or ONX-0912 in patients with DLBCL have not been published.
PI Combination Therapy for DLBCL
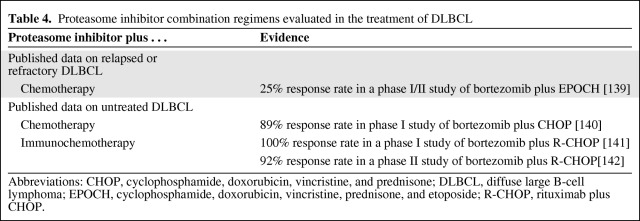
Table 4 summarizes published results of PI combinations for DLBCL. In patients with untreated DLBCL, the addition of bortezomib to CHOP resulted in an ORR of 89% [140], whereas in another phase I study that included 40 patients with untreated DLBCL, the addition of bortezomib to R-CHOP resulted in objective response in all patients with DLBCL, including CRs in 86% of patients [141]. In a randomized, phase II study of 49 patients with aggressive or indolent NHL (including nine with DLBCL and 11 with FL, among others), bortezomib—either once-weekly (1.3 mg/m2 or 1.6 mg/m2) or twice-weekly (1.0 mg/m2 or 1.3 mg/m2)—was administered with R-CHOP for up to six cycles [142]. Most patients (92%) responded to the combination, including CRs in 83% of patients with aggressive NHL, but severe neuropathy was seen among patients who received the higher doses of bortezomib.
Table 4.
Proteasome inhibitor combination regimens evaluated in the treatment of DLBCL
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; DLBCL, diffuse large B-cell lymphoma; EPOCH, cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide; R-CHOP, rituximab plus CHOP.
A study of bortezomib combined with EPOCH in patients with relapsed or refractory DLBCL showed modest activity overall [139]. Subtype analysis in DLBCL patients may help to identify appropriate candidates for combination treatment with a PI. Data suggest that the combination of bortezomib with R-EPOCH shows a dramatic response in the ABC subtype and only 13% in the GCB subtype [143]. A large, randomized study (PYRAMID) is under way to evaluate R-CHOP with and without bortezomib in patients with newly diagnosed DLBCL, and all patients will be screened to identify only those patients with non-GCB subtypes for treatment [144]. The results of that study and other ongoing studies of PI combinations should aid the understanding of the role of these combinations for DLBCL.
Other Types of NHL
Many of the early studies that predominantly evaluated patients with MCL, FL, or DLBCL included a few patients with other types of NHL as well, but the sample sizes generally were too small to draw conclusions about the activity of PI therapy for these NHL subtypes. Activity with PI combination regimens was observed in patients with marginal zone lymphoma or Waldenström's macroglobulinemia (WM). Among patients with WM, the overall response rates were 96% with BORID [145] and 90% with bortezomib plus rituximab [146], and the combination of bortezomib and rituximab plus dexamethasone may be particularly effective for WM, with greater activity than in other types of NHL [100]. Bortezomib combinations appear to have limited activity for chronic or small lymphocytic leukemia; preclinical data suggest that marizomib may be even more effective for this tumor type [25]. Among patients with marginal zone lymphoma, twice-weekly bortezomib plus once-weekly rituximab was associated with response in approximately half of the patients [121].
Bortezomib also induces apoptosis in peripheral T-cell lymphoma (PTCL) cells [13], and phase I studies reported activity in PTCL patients when bortezomib was combined with pegylated liposomal doxorubicin [86], CHOP [147], gemcitabine [148], and both liposomal doxorubicin and gemcitabine [149]. A phase II study of bortezomib with ACVBP resulted in an ORR of 45% in patients with PTCL, but the investigators reported that this was similar to previously reported rates with ACVBP alone [150]. Bortezomib also has single-agent activity in patients with cutaneous T-cell lymphoma (CTCL) [151] and preclinical data suggest that it is synergistic with pralatrexate [152] for CTCL.
Rituximab has been shown to improve outcomes in post-transplant lymphoproliferative disorder caused by Epstein-Barr virus (EBV) [153], and bortezomib has shown activity in EBV-transformed B cells [154]. The combination of bortezomib and rituximab is being evaluated for this condition.
Immunoproteasome Inhibitors
The immunoproteasome is a variant found predominantly in cells of hematopoietic origin. Thus, selectively targeting the immunoproteasome may improve the ratio between antitumor activity and side effects [155]. Immunoproteasome inhibition is being evaluated for multiple myeloma and NHL [26, 156]. This approach is still in the early stages of development and clinical studies of combination treatment with immunoproteasome inhibitors for NHL are not yet available.
Tolerability
The tolerability of bortezomib in combination with other agents (supplemental online Table A) is generally consistent with the most common toxicities of bortezomib monotherapy for NHL (neuropathy, neutropenia, thrombocytopenia, gastrointestinal events, and fatigue) [5, 157, 158] and the toxicities commonly associated with the other agents that are combined with bortezomib. Some studies have suggested that weekly dosing of bortezomib (instead of twice weekly) in combination regimens may improve tolerability [108, 121, 159, 160].
Peripheral sensory neuropathy is a known toxicity of bortezomib, but it is usually reversible [161]. The prescribing information for bortezomib includes detailed instructions about dose reduction, withholding, and discontinuation in patients with moderate-to-severe peripheral neuropathy and/or neuropathic pain. In addition, a recent phase III noninferiority trial comparing i.v. with s.c. administration of bortezomib in patients with multiple myeloma revealed similar efficacies with significantly lower rates of grade ≥3 peripheral neuropathy (16% versus 6%, respectively) and all grade peripheral neuropathy (53% versus 38%, respectively) [162].
Because the other PIs have not been studied as extensively as bortezomib, and because they are not yet available for widespread clinical use, their safety profiles are not as well known. However, as a result of their different chemical properties, it is likely that carfilzomib, marizomib, ONX-0912, and other investigational PIs will have different efficacy and safety profiles from those of bortezomib [34].
Dosing
The recommended dose of bortezomib for relapsed MCL is in 3-week cycles, with an i.v. bolus of bortezomib administered over 3–5 seconds on days 1, 4, 8, and 11 (i.e., twice weekly). Additionally, the s.c. formulation of bortezomib was recently approved with the same dosing guidelines as the i.v. formulation. After the first eight doses, extended therapy can be administered in 5-week cycles on days 1, 8, 15, and 22 (i.e., once weekly). Many different once-weekly and twice-weekly dosing schedules have been evaluated in clinical studies of bortezomib for NHL, as discussed in the sections above. Randomized comparisons generally reported similar efficacies for the twice-weekly schedule and the once-weekly schedule [100, 108, 119, 121, 159, 160]. But, as noted above, weekly dosing of bortezomib in combination regimens led to better tolerability than with the twice-weekly dosing schedule in some of those studies [108, 121, 159, 160]. In our practice, we typically administer bortezomib as a single agent, either i.v. or s.c., using the twice-weekly approved dosing schedule.
Conclusions
Single-agent bortezomib is approved for use for MCL in many countries and has been shown to have activity for other forms of NHL as well. Early clinical studies of other PIs such as carfilzomib and marizomib have not yet provided compelling support for their use as single agents for NHL, but more mature data are needed before their single-agent safety and activity can be determined. The mechanisms of action of PIs are distinct from those of other treatments for NHL, and preclinical evidence suggests that they can be additive or synergistic with many standard treatments for NHL, including biologicals, with an acceptable safety profile.
Early clinical evidence supports the activity of bortezomib combinations for B-cell NHL, particularly when combined with standard treatments such as rituximab and R-CHOP. Controlled clinical trials and long-term data are anticipated to confirm the safety and efficacy of these combinations and PI combinations with other standard treatments for NHL. Combinations of PIs with other novel or targeted agents also have demonstrated additive or synergistic activity in early studies.
Numerous single-arm and controlled studies are under way (supplemental online Table A) that are likely to provide additional evidence to support combinations of bortezomib or other PIs with both standard and emerging treatments for NHL. The results of those studies should serve to guide future research toward finding the best combination therapies for each of these B-cell lymphomas and ultimately extend lives and improve the quality of life of patients.
Recommendations
1. MCL. Bortezomib is the only single agent approved by the U.S. Food and Drug Administration for the treatment of relapsed or refractory MCL, and it remains part of the standard of care in patients with MCL in first or subsequent relapse. Multiple ongoing studies have already shown the feasibility of combining bortezomib with conventional regimens for MCL (including R–hyper-CVAD), and ongoing studies will help refine how to best integrate bortezomib into the management of MCL patients, in both the frontline and salvage settings.
2. FL. Recent data exploring the use of bortezomib as a single agent or in combination therapy with rituximab for FL does not support a change in clinical practice. However, studies of bendamustine-based combinations are still ongoing, with promising preliminary results (a high CR rate).
3. DLBCL. Recent research suggests that preferential inhibition of the NF-κB pathway by bortezomib explains the higher activity seen in combination with immunochemotherapy in patients with the ABC subtype of DLBCL (which is more dependent on NF-κB than the GCB subtype of DLBCL). This might provide a venue for improvement in the outcomes of patients with ABC DLBCL (still worse than those of patients with the GCB subtype treated with R-CHOP), which is currently being tested in a large randomized study (PYRAMID trial).
4. WM. The combination of bortezomib plus rituximab is highly active in patients with WM. This combination should be considered in clinical practice in the relapsed or refractory setting and frontline setting, as shown, for example, in a study that looked at maintenance with bortezomib, dexamethasone, and rituximab every 3 months after induction [145].
5. An s.c. formulation of bortezomib was recently approved with the same dosing guidelines as the i.v. formulation to facilitate administration.
6. Given the number of ongoing trials and options currently available, we highly encourage The Oncologist readership to consider enrollment in clinical trials examining the role of PIs across the spectrum of NHL subtypes to continue to improve patient outcomes.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
During the development of this publication, Jonathan Latham of PharmaScribe, LLC provided editorial assistance with funding from Millennium Pharmaceuticals, Inc.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Anthony R. Mato, Tatyana Feldman, André Goy
Data analysis and interpretation: Anthony R. Mato, Tatyana Feldman, André Goy
Manuscript writing: Anthony R. Mato, Tatyana Feldman, André Goy
Final approval of manuscript: Anthony R. Mato, Tatyana Feldman, André Goy
References
- 1.American Cancer Society. Atlanta: American Cancer Society; 2012. Cancer Facts & Figures 2008; pp. 1–64. [Google Scholar]
- 2.National Cancer Institute. Bethesda, MD: National Cancer Institute; [accessed April 16, 2012]. SEER Stat Fact Sheets–Non-Hodgkin Lymphoma. seer.cancer.gov/statfacts/html/nhl.html. [Google Scholar]
- 3.Ansell SM, Armitage J. Non-Hodgkin lymphoma: Diagnosis and treatment. Mayo Clin Proc. 2005;80:1087–1097. doi: 10.4065/80.8.1087. [DOI] [PubMed] [Google Scholar]
- 4.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goy A, Feldman T. Expanding therapeutic options in mantle cell lymphoma. Clin Lymphoma Myeloma. 2007;7(suppl 5):S184–S191. doi: 10.3816/clm.2007.s.021. [DOI] [PubMed] [Google Scholar]
- 6.Prichard M, Harris T, Williams ME, et al. Treatment strategies for relapsed and refractory aggressive non-Hodgkin's lymphoma. Expert Opin Pharmacother. 2009;10:983–995. doi: 10.1517/14656560902895715. [DOI] [PubMed] [Google Scholar]
- 7.Gisselbrecht C, Vose J, Nademanee A, et al. Radioimmunotherapy for stem cell transplantation in non-Hodgkin's lymphoma: In pursuit of a complete response. The Oncologist. 2009;14(suppl 2):41–51. doi: 10.1634/theoncologist.2009-S2-41. [DOI] [PubMed] [Google Scholar]
- 8.Adams J. The proteasome: A suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 9.Pham LV, Tamayo AT, Yoshimura LC, et al. Inhibition of constitutive NF-κ B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171:88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 10.Strauss SJ, Higginbottom K, Jl̈iger S, et al. The proteasome inhibitor bortezomib acts independently of p53 and induces cell death via apoptosis and mitotic catastrophe in B-cell lymphoma cell lines. Cancer Res. 2007;67:2783–2790. doi: 10.1158/0008-5472.CAN-06-3254. [DOI] [PubMed] [Google Scholar]
- 11.Matta H, Chaudhary PM. The proteasome inhibitor bortezomib (PS-341) inhibits growth and induces apoptosis in primary effusion lymphoma cells. Cancer Biol Ther. 2005;4:77–82. doi: 10.4161/cbt.4.1.1379. [DOI] [PubMed] [Google Scholar]
- 12.Satou Y, Nosaka K, Koya Y, et al. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia. 2004;18:1357–1363. doi: 10.1038/sj.leu.2403400. [DOI] [PubMed] [Google Scholar]
- 13.Nasr R, El-Sabban ME, Karam JA, et al. Efficacy and mechanism of action of the proteasome inhibitor PS-341 in T-cell lymphomas and HTLV-I associated adult T-cell leukemia/lymphoma. Oncogene. 2005;24:419–430. doi: 10.1038/sj.onc.1208212. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Au WY, Guo T, et al. Proteasome inhibitor bortezomib-induced apoptosis in natural killer (NK)-cell leukemia and lymphoma: An in vitro and in vivo preclinical evaluation. Blood. 2007;110:469–470. doi: 10.1182/blood-2007-02-072900. [DOI] [PubMed] [Google Scholar]
- 15.Bonvini P, Zorzi E, Basso G, et al. Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma. Leukemia. 2007;21:838–842. doi: 10.1038/sj.leu.2404528. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Galàn P, Roué G, Villamor N, et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 17.Kabore AF, Sun J, Hu X, et al. The TRAIL apoptotic pathway mediates proteasome inhibitor induced apoptosis in primary chronic lymphocytic leukemia cells. Apoptosis. 2006;11:1175–1193. doi: 10.1007/s10495-006-8048-9. [DOI] [PubMed] [Google Scholar]
- 18.Rizzatti EG, Mora-Jensen H, Weniger MA, et al. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-κB pathways. Leuk Lymphoma. 2008;49:798–808. doi: 10.1080/10428190801910912. [DOI] [PubMed] [Google Scholar]
- 19.Ishii Y, Pirkmaier A, Alvarez JV, et al. Cyclin D1 overexpression and response to bortezomib treatment in a breast cancer model. J Natl Cancer Inst. 2006;98:1238–1247. doi: 10.1093/jnci/djj334. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor OA, Smith EA, Toner LE, et al. The combination of the proteasome inhibitor bortezomib and the Bcl-2 antisense molecule oblimersen sensitizes human B-cell lymphomas to cyclophosphamide. Clin Cancer Res. 2006;12:2902–2911. doi: 10.1158/1078-0432.CCR-05-0308. [DOI] [PubMed] [Google Scholar]
- 21.Labussière M, Pinel S, Vandamme M, et al. Radiosensitizing properties of bortezomib depend on therapeutic schedule. Int J Radiat Oncol Biol Phys. 2011;79:892–900. doi: 10.1016/j.ijrobp.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 22.Kamer S, Ren Q, Dicker AP. Differential radiation sensitization of human cervical cancer cell lines by the proteasome inhibitor Velcade (bortezomib, PS-341) Arch Gynecol Obstet. 2009;279:41–46. doi: 10.1007/s00404-008-0667-7. [DOI] [PubMed] [Google Scholar]
- 23.Ahn KS, Sethi G, Chao TH, et al. Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-κB regulated gene products. Blood. 2007;110:2286–2295. doi: 10.1182/blood-2007-04-084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor OA, Stewart AK, Vallone M, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz S, Krupnik Y, Keating M, et al. The proteasome inhibitor NPI-0052 is a more effective inducer of apoptosis than bortezomib in lymphocytes from patients with chronic lymphocytic leukemia. Mol Cancer Ther. 2006;5:1836–1843. doi: 10.1158/1535-7163.MCT-06-0066. [DOI] [PubMed] [Google Scholar]
- 26.Roccaro AM, Sacco A, Aujay M, et al. Selective inhibition of chymotrypsin-like activity of the immunoproteasome and constitutive proteasome in Waldenstrom macroglobulinemia. Blood. 2010;115:4051–4060. doi: 10.1182/blood-2009-09-243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupperman E, Lee EC, Cao Y, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan D, Hideshima T, Anderson KC. A novel proteasome inhibitor NPI-0052 as an anticancer therapy. Br J Cancer. 2006;95:961–965. doi: 10.1038/sj.bjc.6603406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson MJ, Blank JL, Bruzzese FJ, et al. Comparison of biochemical and biological effects of ML858 (salinosporamide A) and bortezomib. Mol Cancer Ther. 2006;5:3052–3061. doi: 10.1158/1535-7163.MCT-06-0185. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Miller CP, Ban K, Dujka ME, et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110:267–277. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potts BC, Albitar MX, Anderson KC, et al. Marizomib, a proteasome inhibitor for all seasons: Preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11:254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dick LR, Fleming PE. Building on bortezomib: Second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov Today. 2010;15:243–249. doi: 10.1016/j.drudis.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan D, Singh AV, Aujay M, et al. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood. 2010;116:4906–4915. doi: 10.1182/blood-2010-04-276626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dreyling M. ESMO Guidelines Working Group. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v181–v183. doi: 10.1093/annonc/mdq184. [DOI] [PubMed] [Google Scholar]
- 37.Tilly H, Dreyling M. ESMO Guidelines Working Group. Diffuse large B-cell non-Hodgkin's lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v172–v174. doi: 10.1093/annonc/mdq203. [DOI] [PubMed] [Google Scholar]
- 38.Weigert O, Pastore A, Rieken M, et al. Sequence-dependent synergy of the proteasome inhibitor bortezomib and cytarabine in mantle cell lymphoma. Leukemia. 2007;21:524–528. doi: 10.1038/sj.leu.2404511. [DOI] [PubMed] [Google Scholar]
- 39.Kapanen A, Tucker C, Chikh G, et al. Cell based assays completed with the mantle cell lymphoma cell lines Z138 and NCEB-1 indicate that combinations of bortezomib and flavopiridol interact to achieve synergistic activity [abstract] Blood. 2005;106 Abstract 2410. [Google Scholar]
- 40.Wadehra N, Lin T, Ryan T, et al. Combination bortezomib (PS341, Velcade) and rituximab treatment affects multiple survival and death pathways to promote apoptosis in mantle cell lymphoma [abstract] Blood. 2005;106 doi: 10.4161/mabs.1.1.7472. Abstract 2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Han XH, Zhang L, et al. Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia. 2008;22:179–185. doi: 10.1038/sj.leu.2404959. [DOI] [PubMed] [Google Scholar]
- 42.Alinari L, White VL, Earl CT, et al. Combination bortezomib and rituximab treatment affects multiple survival and death pathways to promote apoptosis in mantle cell lymphoma. MAbs. 2009;1:31–40. doi: 10.4161/mabs.1.1.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor O, Toner L, Smith E, et al. Combined targeting of the proteasome and Bcl-2 sensitizes human B-cell lymphoma and multiple myeloma to cyclophosphamide in in vitro and in vivo models of these diseases [abstract] Blood. 2004;104 Abstract 2484. [Google Scholar]
- 44.Yazbeck VY, Georgakis GV, Li Y, et al. Inhibition of the Pan-Bcl-2 family by the small molecule GX15–070 induces apoptosis in mantle cell lymphoma (MCL) cells and enhances the activity of two proteasome inhibitors (NPI-0052 and bortezomib), and doxorubicin chemotherapy [abstract] Blood. 2006;108 Abstract 2532. [Google Scholar]
- 45.Shore G, Watson M, Roulston A, et al. Obatoclax (GX15–070) is a potent antagonist of constitutive Mcl-1/Bak interactions in intact mitochondrial membrane and synergizes with bortezomib in mantle cell lymphoma [abstract] Blood. 2006;108 Abstract 832. [Google Scholar]
- 46.Gruber EA, Czuczman MS, Olejniczak SH, et al. GX15–070 and bortezomib induce up-regulation of BH3 single domain pro-apoptotic proteins Puma and Noxa and is associated with synergistic anti-tumor activity in rituximab-sensitive, rituximab-resistant cell lines (RSCL and RRCL), and primary lymphoma patient specimens [abstract] Blood. 2007;110 Abstract 1389. [Google Scholar]
- 47.Pérez-Galàn P, Roué G, Villamor N, et al. The BH3-mimetic GX15–070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 48.Pérez-Galàn P, Roué G, López-Guerra M, et al. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15–070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia. 2008;22:1712–1720. doi: 10.1038/leu.2008.175. [DOI] [PubMed] [Google Scholar]
- 49.Paoluzzi L, Gonen M, Bhagat G, et al. The BH3-only mimetic ABT-737 synergizes the anti-neoplastic activity of proteasome inhibitors in lymphoid malignancies. Blood. 2008;112:2906–2916. doi: 10.1182/blood-2007-12-130781. [DOI] [PubMed] [Google Scholar]
- 50.Antun A, Cerchietti L, Aparo S, et al. BCL6 inhibitor peptide have powerful anti-lymphoma activity in animal models of diffuse large B-cell lymphoma and synergize with other anti-lymphoma drugs [abstract] Blood. 2006;108 Abstract 827. [Google Scholar]
- 51.Ackler S, Mitten MJ, Foster K, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010;66:869–880. doi: 10.1007/s00280-009-1232-1. [DOI] [PubMed] [Google Scholar]
- 52.Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-κB-dependent process. Oncogene. 2003;22:7108–7122. doi: 10.1038/sj.onc.1206863. [DOI] [PubMed] [Google Scholar]
- 53.Ismail LK, Timm M, Novak A, et al. Rapamycin enhances the cytotoxicity of bortezomib and rituximab on mantle cell lymphoma (MCL) cell lines [abstract] Blood. 2005;106 Abstract 2411. [Google Scholar]
- 54.Haritunians T, Mori A, O'Kelly J, et al. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 55.Maharaj L, Popat R, Chahwan A, et al. The novel HDAC inhibitor UCL67022 is highly potent in multiple myeloma and non-Hodgkin's lymphoma and is enhanced by bortezomib [abstract] Blood. 2006;108 Abstract 2604. [Google Scholar]
- 56.Bhalla S, David K, Mauro L, et al. Histone deacetylase inhibitor (HDACi) PCI-24781 and bortezomib result in synergistic apoptosis in Hodgkin lymphoma (HL) and non-Hodgkin's lymphoma (NHL) cell lines: Investigation of cell death and NFκB-mediated pathways [abstract] Blood. 2007;110 Abstract 3589. [Google Scholar]
- 57.Heider U, von Metzler I, Kaiser M, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol. 2008;80:133–142. doi: 10.1111/j.1600-0609.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 58.Rao R, Fiskus W, Joshi R, et al. Targeting histone deacetylases and unfolded protein mediated endoplasmic reticulum (ER) stress as a strategy against human mantle cell lymphoma [abstract] Blood. 2007;110 Abstract 1378. [Google Scholar]
- 59.Paoluzzi L, Scotto L, Marchi E, et al. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin Cancer Res. 2010;16:554–565. doi: 10.1158/1078-0432.CCR-09-1937. [DOI] [PubMed] [Google Scholar]
- 60.Hernandez-Ilizaliturri FJ, Mavis C, Maraj I, et al. Panobinostat, a novel histone deacetylase (HiDAC) inhibitor enhances the anti-tumor activity of bortezomib (BTZ) in rituximab-chemotherapy sensitive and resistant lymphoma cell lines [abstract] Blood. 2010;116 Abstract 3936. [Google Scholar]
- 61.Rao R, Fiskus W, Balusu R, et al. Treatment with histone deacetylase 6-specific inhibitor WT-161 disrupts hsp90 function, abrogates aggresome formation and sensitizes human mantle cell lymphoma cells to lethal ER stress induced by proteasome inhibitor carfilzomib [abstract] Blood. 2010;116 Abstract 2856. [Google Scholar]
- 62.Dasmahapatra G, Lembersky D, Friedberg JW, et al. Histone deacetylase inhibitors potentiate the lethality of the irreversible proteasome inhibitor carfilzomib in mantle cell lymphoma cells in vitro and in vivo [abstract] Blood. 2010;116 Abstract 3938. [Google Scholar]
- 63.Paoluzzi L, Scotto L, Marchi E, et al. The anti-histaminic cyproheptadine synergizes the antineoplastic activity of bortezomib in mantle cell lymphoma through its effects as a histone deacetylase inhibitor. Br J Haematol. 2009;146:656–659. doi: 10.1111/j.1365-2141.2009.07797.x. [DOI] [PubMed] [Google Scholar]
- 64.Rao R, Nalluri S, Fiskus W, et al. Role of CAAT/enhancer binding protein homologous protein in panobinostat-mediated potentiation of bortezomib-induced lethal endoplasmic reticulum stress in mantle cell lymphoma cells. Clin Cancer Res. 2010;16:4742–4754. doi: 10.1158/1078-0432.CCR-10-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia X, Leleu X, Moreau AS, et al. The proteasome inhibitor NPI-0052 in combination with bortezomib induces antitumor activity in Waldenstrom macroglobulinemia [abstract] Blood. 2006;108 Abstract 4746. [Google Scholar]
- 66.Roccaro A, Leleu X, Sacco A, et al. The combination of bortezomib and NPI-0052 exerts anti-tumor activity in Waldenstrom macroglobulinemia (WM) [abstract] Blood. 2007;110 Abstract 1516. [Google Scholar]
- 67.Leleu X, O'Sullivan G, Jia X, et al. Novel agent perifosine enhances antitumor activity of bortezomib, rituximab and other conventional therapies in Waldenstrom's macroglobulinemia [abstract] Blood. 2006;108 Abstract 2517. [Google Scholar]
- 68.Moreau AS, Jia X, O'Sullivan G, et al. The selective protein kinase CB inhibitor, enzastaurin, induces in vitro and in vivo antitumor activity in Waldenstrom's macroglobulinemia [abstract] Blood. 2006;108 doi: 10.1182/blood-2006-10-054577. Abstract 2496. [DOI] [PubMed] [Google Scholar]
- 69.Pham LV, Tamayo AT, Li C, et al. Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: Therapeutic implications. Mol Cancer Ther. 2010;9:2026–2036. doi: 10.1158/1535-7163.MCT-10-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roué G, Pérez-Galàn P, Mozos A, et al. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood. 2011;117:1270–1279. doi: 10.1182/blood-2010-04-278853. [DOI] [PubMed] [Google Scholar]
- 71.Busse A, Kraus M, Na IK, et al. Sensitivity of tumor cells to proteasome inhibitors is associated with expression levels and composition of proteasome subunits. Cancer. 2008;112:659–670. doi: 10.1002/cncr.23224. [DOI] [PubMed] [Google Scholar]
- 72.Pervan M, Calimlim J, Matso D, et al. Active combination therapy of bortezomib (Velcade) and ibritumomab tiuxetan (Zevalin) in an in vivo diffuse large B-cell lymphoma model [abstract] Blood. 2005;106 Abstract 2406. [Google Scholar]
- 73.Roué G, Pérez-Galàn P, López-Guerra M, et al. Selective inhibition of IκB kinase sensitizes mantle cell lymphoma B cells to TRAIL by decreasing cellular FLIP level. J Immunol. 2007;178:1923–1930. doi: 10.4049/jimmunol.178.3.1923. [DOI] [PubMed] [Google Scholar]
- 74.Georgakis GV, Li Y, Humphreys R, et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: Induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol. 2005;130:501–510. doi: 10.1111/j.1365-2141.2005.05656.x. [DOI] [PubMed] [Google Scholar]
- 75.Smith MR, Jin F, Joshi I. Bortezomib sensitizes non-Hodgkin's lymphoma cells to apoptosis induced by antibodies to tumor necrosis factor related apoptosis-inducing ligand (TRAIL) receptors TRAIL-R1 and TRAIL-R2. Clin Cancer Res. 2007;13:5528s–5534s. doi: 10.1158/1078-0432.CCR-07-0982. [DOI] [PubMed] [Google Scholar]
- 76.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with rituximab-hyperCVAD alternating with rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 77.Evens AM, Winter JN, Hou N, et al. A phase II clinical trial of intensive chemotherapy followed by consolidative stem cell transplant: Long-term follow-up in newly diagnosed mantle cell lymphoma. Br J Haematol. 2008;140:385–393. doi: 10.1111/j.1365-2141.2007.06908.x. [DOI] [PubMed] [Google Scholar]
- 78.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 80.Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4473–4479. doi: 10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- 81.Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 82.Jermann M, Jost LM, Taverna C, et al. Rituximab-EPOCH, an effective salvage therapy for relapsed, refractory or transformed B-cell lymphomas: Results of a phase II study. Ann Oncol. 2004;15:511–516. doi: 10.1093/annonc/mdh093. [DOI] [PubMed] [Google Scholar]
- 83.Morschhauser F, Dreyling M, Rohatiner A, et al. Rationale for consolidation to improve progression-free survival in patients with non-Hodgkin's lymphoma: A review of the evidence. The Oncologist. 2009;14(suppl 2):17–29. doi: 10.1634/theoncologist.2009-S2-17. [DOI] [PubMed] [Google Scholar]
- 84.Jäeger G, Neumeister P, Brezinschek R, et al. Rituximab (anti-CD20 monoclonal antibody) as consolidation of first-line CHOP chemotherapy in patients with follicular lymphoma: A phase II study. Eur J Haematol. 2002;69:21–26. doi: 10.1034/j.1600-0609.2002.01692.x. [DOI] [PubMed] [Google Scholar]
- 85.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 86.Orlowski RZ, Voorhees PM, Garcia RA, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- 87.O'Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 88.Strauss SJ, Maharaj L, Hoare S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: Potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24:2105–2112. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- 89.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 90.Faderl S, Rai K, Gribben J, et al. Phase II study of single-agent bortezomib for the treatment of patients with fludarabine-refractory B-cell chronic lymphocytic leukemia. Cancer. 2006;107:916–924. doi: 10.1002/cncr.22097. [DOI] [PubMed] [Google Scholar]
- 91.Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: The National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol. 2007;18:116–121. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 92.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 93.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: Updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O'Connor OA, Moskowitz C, Portlock C, et al. Patients with chemotherapy-refractory mantle cell lymphoma experience high response rates and identical progression-free survivals compared with patients with relapsed disease following treatment with single agent bortezomib: Results of a multicentre phase 2 clinical trial. Br J Haematol. 2009;145:34–39. doi: 10.1111/j.1365-2141.2008.07466.x. [DOI] [PubMed] [Google Scholar]
- 95.Goy A, Bernstein SH, McDonald A, et al. Potential biomarkers of bortezomib activity in mantle cell lymphoma from the phase 2 PINNACLE trial. Leuk Lymphoma. 2010;51:1269–1277. doi: 10.3109/10428194.2010.483302. [DOI] [PubMed] [Google Scholar]
- 96.Alsina M, Trudel S, Vallone M, et al. Phase 1 single agent antitumor activity of twice weekly consecutive day dosing of the proteasome inhibitor carfilzomib (PR-171) in hematologic malignancies [abstract] Blood. 2007;110 Abstract 411. [Google Scholar]
- 97.Orlowski RZ, Stewart K, Vallone M, et al. Safety and antitumor efficacy of the proteasome inhibitor carfilzomib (PR-171) dosed for five consecutive days in hematologic malignancies: Phase 1 results [abstract] Blood. 2007;110 Abstract 409. [Google Scholar]
- 98.Aghajanian CA, Hamlin P, Gordon MS, et al. Phase I study of the novel proteasome inhibitor NPI-0052 in patients with lymphoma and solid tumors. J Clin Oncol. 2008;26(15 suppl) Abstract 3574. [Google Scholar]
- 99.Baiocchi RA, Alinari L, Lustberg ME, et al. Phase 2 trial of rituximab and bortezomib in patients with relapsed or refractory mantle cell and follicular lymphoma. Cancer. 2011;117:2355–2581. doi: 10.1002/cncr.25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agathocleous A, Rohatiner A, Rule S, et al. Weekly versus twice weekly bortezomib given in conjunction with rituximab, in patients with recurrent follicular lymphoma, mantle cell lymphoma and Waldenström macroglobulinaemia. Br J Haematol. 2010;151:346–353. doi: 10.1111/j.1365-2141.2010.08340.x. [DOI] [PubMed] [Google Scholar]
- 101.Chiappella A, Pregno P, Zinzani PL, et al. Weekly infusion of bortezomib in combination with rituximab in relapsed/refractory indolent non-follicular and mantle cell lymphoma is safe and effective: Two-years analysis of phase II trial BRIL06 of Intergruppo Italiano Linfomi (IIL) [abstract] Blood. 2010;116 Abstract 3965. [Google Scholar]
- 102.Morrison VA, Johnson JL, Jung S, et al. A phase II trial of bortezomib plus lenalidomide for relapsed/refractory mantle cell lymphoma (MCL) (CALGB 50501): Results of a planned interim analysis. J Clin Oncol. 2010;28(15 suppl) Abstract 8106. [Google Scholar]
- 102a.Beaven AW, Shea TC, Moore DT, et al. A phase I study evaluating ibritumomab tiuxetan (Zevalin®) in combination with bortezomib (Velcade®) in relapsed/refractory mantle cell and low grade B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2012;53:254–258. doi: 10.3109/10428194.2011.608445. [DOI] [PubMed] [Google Scholar]
- 103.Snell M, Koc ON, Bahlis NJ, et al. A phase I trial of PS-341 and fludarabine for relapsed and refractory indolent non-Hodgkin's lymphoma and chronic lymphocytic leukemia. J Clin Oncol. 2006;24(18 suppl) Abstract 7580. [Google Scholar]
- 104.Weigert O, Weidmann E, Mueck R, et al. A novel regimen combining high dose cytarabine and bortezomib has activity in multiply relapsed and refractory mantle cell lymphoma—long-term results of a multicenter observation study. Leuk Lymphoma. 2009;50:716–722. doi: 10.1080/10428190902856790. [DOI] [PubMed] [Google Scholar]
- 105.Kouroukis CT, Fernandez LA, Crump M, et al. A phase II study of bortezomib and gemcitabine in relapsed mantle cell lymphoma from the National Cancer Institute of Canada Clinical Trials Group (IND 172) Leuk Lymphoma. 2011;52:394–399. doi: 10.3109/10428194.2010.546015. [DOI] [PubMed] [Google Scholar]
- 106.Orciuolo E, Buda G, Pelosini M, et al. Fludarabine, bortezomib, Myocet and rituximab chemotherapy in relapsed and refractory mantle cell lymphoma. Br J Haematol. 2010;148:810–812. doi: 10.1111/j.1365-2141.2009.07998.x. [DOI] [PubMed] [Google Scholar]
- 107.Musto PM, Guariglia R, Pietrantuono G, et al. A pilot study with rituximab, bortezomib and hyper-fractionated cyclophosphamide (RBC regimen) for the treatment of advanced mantle cell lymphoma in elderly patients. Haematologica. 2008;93(suppl 1) Abstract 0273. [Google Scholar]
- 108.Gerecitano J, Portlock CS, Hamlin PA, Jr, et al. Rituximab, cyclophosphamide, bortezomib and prednisone (R-CBorP): Final results of a phase I trial evaluating two dosing schedules and the safety of overlapping pegfilgrastim in patients with relapsed/refractory indolent and mantle cell lymphomas [abstract] Blood. 2009;114 Abstract 3708. [Google Scholar]
- 109.Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–2812. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drach J, Kaufmann H, Pichelmayer O, et al. Bortezomib, rituximab, and dexamethasone (BORID) as salvage treatment in relapsed/refractory mantle cell lymphoma: Sustained disease control in patients achieving a complete remission [abstract] Blood. 2007;110 Abstract 2578. [Google Scholar]
- 111.Gressin R, Houot R, Uribe MO, et al. Final results of the RiPAD+C regimen including Velcade in front line therapy for elderly patients with mantle cell lymphoma. A phase II prospective study of the GOELAMS group [abstract] Blood. 2010;116 Abstract 2791. [Google Scholar]
- 112.Romaguera J, Fayad L, McLaughlin P, et al. Phase I trial of bortezomib in combination with rituximab-hyperCVAD/methotrexate and cytarabine for untreated mantle cell lymphoma [abstract] Blood. 2008;112 doi: 10.1111/j.1365-2141.2010.08315.x. Abstract 3051. [DOI] [PubMed] [Google Scholar]
- 113.Romaguera JE, Fayad LE, McLaughlin P, et al. Phase I trial of bortezomib in combination with rituximab-hyperCVAD alternating with rituximab, methotrexate and cytarabine for untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;151:47–53. doi: 10.1111/j.1365-2141.2010.08315.x. [DOI] [PubMed] [Google Scholar]
- 114.Kahl B, Chang J, Eickhoff J, et al. VcR-CVAD produces a high complete response rate in untreated mantle cell lymphoma: A phase II study from the Wisconsin oncology network [abstract] Blood. 2008;112 Abstract 265. [Google Scholar]
- 115.Goy A, Ford P, Feldman T, et al. A phase 1 trial of the pan Bcl-2 family inhibitor obatoclax mesylate (GX15–070) in combination with bortezomib in patients with relapsed/refractory mantle cell lymphoma [abstract] Blood. 2007;110 Abstract 2569. [Google Scholar]
- 116.Friedberg JW. Treatment of follicular non-Hodgkin's lymphoma: The old and the new. Semin Hematol. 2008;45(suppl 2):S2–S6. doi: 10.1053/j.seminhematol.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paoluzzi L, O'Connor OA. Mechanistic rationale and clinical evidence for the efficacy of proteasome inhibitors against indolent and mantle cell lymphomas. BioDrugs. 2006;20:13–23. doi: 10.2165/00063030-200620010-00002. [DOI] [PubMed] [Google Scholar]
- 118.Di Bella N, Taetle R, Kolibaba K, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115:475–480. doi: 10.1182/blood-2009-08-233155. [DOI] [PubMed] [Google Scholar]
- 119.Ribrag V, Tilly H, Casasnovas O, et al. Final results of a randomized phase 2 multicenter study of two bortezomib schedules in patients with recurrent or refractory follicular lymphoma. Groupe d'Etude des Lymphomes de l'Adulte (GELA) study FL-05 [abstract] Blood. 2010;116 Abstract 768. [Google Scholar]
- 120.O'Connor OA, Portlock C, Moskowitz C, et al. Time to treatment response in patients with follicular lymphoma treated with bortezomib is longer compared with other histologic subtypes. Clin Cancer Res. 2010;16:719–726. doi: 10.1158/1078-0432.CCR-08-2647. [DOI] [PubMed] [Google Scholar]
- 121.de Vos S, Goy A, Dakhil SR, et al. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27:5023–5030. doi: 10.1200/JCO.2008.17.7980. [DOI] [PubMed] [Google Scholar]
- 122.Coiffier B, Osmanov EA, Hong X, et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol. 2011;12:773–784. doi: 10.1016/S1470-2045(11)70150-4. [DOI] [PubMed] [Google Scholar]
- 123.Coiffier B, Li W, Henitz ED, et al. Identification of patient subgroups demonstrating longer progression-free survival (PFS) benefit with bortezomib-rituximab versus rituximab in patients with relapsed or refractory follicular lymphoma (FL): biomarker analyses of the phase 3 LYM3001 study [abstract] Blood. 2011;118 Abstract 265. [Google Scholar]
- 124.Theocharous P, Shpilberg O, Elsayed YA. A randomized, open-label, multicenter phase II study of bortezomib with fludarabine in comparison to rituximab with fludarabine in follicular lymphoma subjects previously treated with rituximab. J Clin Oncol. 2010;28(15 suppl) Abstract TPS303. [Google Scholar]
- 125.Roy R, Evens AM, Patton DP, et al. Bortezomib may be safely combined with y-90 ibritumomab tiuxetan in patients with relapsed/refractory follicular or transformed non-Hodgkin lymphoma: A phase I trial of combined induction therapy and bortezomib consolidation [abstract] Blood. 2010;116 doi: 10.3109/10428194.2012.722215. Abstract 1749. [DOI] [PubMed] [Google Scholar]
- 126.Barr PM, Fu P, Lazarus HM, et al. Phase I trial of fludarabine, bortezomib and rituximab for relapsed and refractory indolent and mantle cell non-Hodgkin lymphoma. Br J Haematol. 2009;147:89–96. doi: 10.1111/j.1365-2141.2009.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedberg JW, Vose JM, Kelly JL, et al. Bendamustine, bortezomib and rituximab in patients (pts) relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma (NHL): A multicenter phase II clinical trial [abstract] Blood. 2009;114 Abstract 924. [Google Scholar]
- 128.Fowler N, Kahl BS, Lee P, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: The phase II VERTICAL study. J Clin Oncol. 2011;29:3389–3395. doi: 10.1200/JCO.2010.32.1844. [DOI] [PubMed] [Google Scholar]
- 129.Craig M, Hanna WT, Cabanillas F, et al. Bortezomib in combination with rituximab, cyclophosphamide, and prednisone with or without doxorubicin followed by rituximab maintenance in patients with relapsed or refractory follicular lymphoma: Results of a phase 2 study [abstract] Blood. 2010;116 doi: 10.1111/bjh.12991. Abstract 2798. [DOI] [PubMed] [Google Scholar]
- 130.Sehn LH, Macdonald D, Rubin S, et al. Bortezomib added to R-CVP is safe and effective for previously untreated advanced-stage follicular lymphoma: A phase II study by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2011;29:3396–3401. doi: 10.1200/JCO.2010.33.6594. [DOI] [PubMed] [Google Scholar]
- 131.Salles G. Is there a role for bortezomib combinations in the management of patients with follicular lymphoma? J Clin Oncol. 2011;29:3349–3350. doi: 10.1200/JCO.2011.35.5586. [DOI] [PubMed] [Google Scholar]
- 132.Nabhan C, Dalal N, Tolzien K, et al. Bortezomib (Velcade), rituximab, cyclophosphamide, dexamethasone (VRCD) combination therapy in front-line low-grade non-Hodgkin lymphoma (LG-NHL) is active in elderly patient population [abstract] Blood. 2010;116 Abstract 1769. [Google Scholar]
- 133.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–2724. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–4594. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 135.Fitoussi O, Belhadj K, Mounier N, et al. Survival impact of rituximab combined with ACVBP and upfront consolidative autotransplantation in high risk diffuse large B-cell lymphoma for GELA. Haematologica. 2011;96:1136–1143. doi: 10.3324/haematol.2010.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Imhoff GW, Boerma EJ, van der Holt B, et al. Prognostic impact of germinal center-associated proteins and chromosomal breakpoints in poor-risk diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:4135–4142. doi: 10.1200/JCO.2006.05.5897. [DOI] [PubMed] [Google Scholar]
- 137.Hong J, Park S, Park J, et al. Evaluation of prognostic values of clinical and histopathologic characteristics in diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy. Leuk Lymphoma. 2011;52:1904–1912. doi: 10.3109/10428194.2011.588761. [DOI] [PubMed] [Google Scholar]
- 138.Milhollen MA, Traore T, Adams-Duffy J, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: Rationale for treatment of NF-κB-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 139.Dunleavy K, Janik J, Gea-Banacloche J, et al. Phase I/II study of bortezomib alone and bortezomib with dose-adjusted EPOCH chemotherapy in relapsed or refractory aggressive B-cell lymphoma [abstract] Blood. 2004;104 Abstract 1385. [Google Scholar]
- 140.Jang G, Sym SJ, Kim S, et al. A phase I trial of bortezomib plus CHOP every 2 weeks in patients with advanced stage diffuse large B-cell lymphomas [abstract] Blood. 2007;110 doi: 10.5045/kjh.2012.47.1.53. Abstract 4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 142.Mounier N, Ribrag V, Haioun C, et al. Efficacy and toxicity of two schedules of R-CHOP plus bortezomib in front-line B lymphoma patients: A randomized phase II trial from the Groupe d'Etude des Lymphomes de l'Adulte (GELA) J Clin Oncol. 2007;25(18 suppl) Abstract 8010. [Google Scholar]
- 143.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Doner K, Flinn IW, Ulrich BK, et al. Rapid prospective identification of non-germinal center B cell-like (GCB) diffuse large B-cell lymphoma (DLBCL) patients for targeted trials: Early results from PYRAMID, a phase 2 randomized study of R-CHOP ± bortezomib in newly diagnosed non-GCB DLBCL [abstract] Blood. 2010;116 Abstract 1792. [Google Scholar]
- 145.Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009;27:3830–3835. doi: 10.1200/JCO.2008.20.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghobrial IM, Matous J, Padmanabhan S, et al. Phase II trial of combination of bortezomib and rituximab in relapsed and/or refractory Waldenstrom macroglobulinemia [abstract] Blood. 2008;112 Abstract 832. [Google Scholar]
- 147.Lee J, Suh C, Kang HJ, et al. Phase I study of proteasome inhibitor bortezomib plus CHOP in patients with advanced, aggressive T-cell or NK/T-cell lymphoma. Ann Oncol. 2008;19:2079–2083. doi: 10.1093/annonc/mdn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Evens AM, Gordon LI, Patton D, et al. Phase I results of combination gemcitabine and bortezomib (Velcade®) for relapsed/refractory nodal T-cell non-Hodgkin lymphoma (T-NHL) and aggressive B-cell NHL (B-NHL) [abstract] Blood. 2008;112 Abstract 2005. [Google Scholar]
- 149.Dhillon N, Bakkannagari S, Ng C, et al. Cutaneous T cell lymphoma: Responses in phase 1 trial of combination therapy with liposomal doxorubicin, bortezomib, and gemcitabine [abstract] Blood. 2006;108 Abstract 2466. [Google Scholar]
- 150.Delmer A, Fitoussi O, Gaulard P, et al. A phase II study of bortezomib in combination with intensified CHOP-like regimen (ACVBP) in patients with previously untreated T-cell lymphoma: Results of the GELA LNH05-1T trial. J Clin Oncol. 2009;27(15 suppl) Abstract 8554. [Google Scholar]
- 151.Zinzani PL, Musuraca G, Tani M, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:4293–4297. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]
- 152.Marchi E, Paoluzzi L, Scotto L, et al. Pralatrexate is synergistic with the proteasome inhibitor bortezomib in in vitro and in vivo models of T-cell lymphoid malignancies. Clin Cancer Res. 2010;16:3648–3658. doi: 10.1158/1078-0432.CCR-10-0671. [DOI] [PubMed] [Google Scholar]
- 153.Styczynski J, Einsele H, Gil L, et al. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: A comprehensive review of reported cases. Transpl Infect Dis. 2009;11:383–392. doi: 10.1111/j.1399-3062.2009.00411.x. [DOI] [PubMed] [Google Scholar]
- 154.Zou P, Kawada J, Pesnicak L, et al. Bortezomib induces apoptosis of Epstein-Barr virus (EBV)-transformed B cells and prolongs survival of mice inoculated with EBV-transformed B cells. J Virol. 2007;81:10029–10036. doi: 10.1128/JVI.02241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuhn DJ, Orlowski RZ, Bjorklund CC. Second generation proteasome inhibitors: Carfilzomib and immunoproteasome-specific inhibitors (IPSIs) Curr Cancer Drug Targets. 2011;11:285–295. doi: 10.2174/156800911794519725. [DOI] [PubMed] [Google Scholar]
- 156.Kuhn DJ, Hunsucker SA, Chen Q, et al. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood. 2009;113:4667–4676. doi: 10.1182/blood-2008-07-171637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Utecht KN, Kolesar J. Bortezomib: A novel chemotherapeutic agent for hematologic malignancies. Am J Health Syst Pharm. 2008;65:1221–1231. doi: 10.2146/ajhp070272. [DOI] [PubMed] [Google Scholar]
- 158.Suh KS, Goy A. Bortezomib in mantle cell lymphoma. Future Oncol. 2008;4:149–168. doi: 10.2217/14796694.4.2.149. [DOI] [PubMed] [Google Scholar]
- 159.Agathocleous A, Rule S, Johnson P, et al. Preliminary results of a phase I/II study of weekly or twice weekly bortezomib in combination with rituximab, in patients with follicular lymphoma, mantle cell lymphoma and Waldenstrom's macroglobulinaemia [abstract] Blood. 2007;110 Abstract 2559. [Google Scholar]
- 160.Moosmann PR, Heizmann M, Kotrubczik N, et al. Weekly treatment with a combination of bendamustine and bortezomib in relapsed or refractory indolent non-Hodgkin's lymphoma: A single-center phase 1/2 study [abstract] Blood. 2008;112 doi: 10.3109/10428190903275602. Abstract 1574. [DOI] [PubMed] [Google Scholar]
- 161.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 162.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 163.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: Perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–762. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.