Anemia is a common manifestation in patients with cancer. The meta-analysis of randomized controlled trials aims to determine the frequency of and risk for anemia with targeted therapies used to treat patients with solid tumors.
Keywords: Anemia, Targeted therapies, Solid tumors, Meta-analysis, Erythropoiesis-stimulating agents
Abstract
Background.
Anemia is a common manifestation in patients with cancer. Little is known about the frequency of and risk for anemia with targeted therapies used to treat solid tumors.
Methods.
We performed a meta-analysis of randomized controlled trials of solid tumors by comparing targeted therapy (alone or in combination) with standard therapy alone to calculate the incidence and relative risk (RR) for anemia events associated with these agents. Overall, 24,310 patients were included in the analysis.
Results.
The addition of targeted therapies to standard treatment (chemotherapy or placebo/best supportive care) increased the risk for all grades of anemia by 7%. The RR for all grades (incidence, 44%) and grades 1–2 (incidence, 38.9%) of anemia was higher with biological therapies alone but not when combined with chemotherapy. The risk was significant for erlotinib, trastuzumab, and sunitinib. Bevacizumab was associated with a lower risk for anemia. Anti–epidermal growth factor receptor, anti–human epidermal growth factor receptor 2, anti–vascular endothelial growth factor receptors, and tyrosine kinase inhibitors predicted RRs of 1.24, 1.20, 0.82, and 1.33, respectively, and all of these values were significant.
Conclusion.
Grade 1–2 anemia is frequently associated with biological agents. The risk is particularly associated with small-molecule tyrosine kinase inhibitors (gefitinib and erlotinib), breast cancer, and lung cancer. Erythropoiesis-stimulating agents are not labeled for use with targeted therapies (without chemotherapy) and the treatment is supportive only.
Introduction
Anemia is a frequent and serious complication experienced by many patients with cancer, especially those receiving chemotherapy. Because of the potential deleterious effects of anemia on a patient's quality of life, performance score, and therapeutic outcome, the treatment of anemia is an important component in the overall care of patients with cancer [1]. Treatment interventions include blood transfusions, iron supplementation, and recombinant human erythropoietin. In particular, three major preparations of recombinant human erythropoietin are used worldwide for the treatment of anemia in patients with cancer: epoetin alfa, epoetin beta, and darbepoetin alfa.
Over the past few years, molecular-targeted therapies have revolutionized the treatment of cancer and have increased the overall survival times of patients with several types of solid tumors, including trastuzumab for breast cancer, bevacizumab for lung and colorectal cancer, sunitinib for kidney cancer, and sorafenib for hepatocellular carcinoma. All these biological agents are invariably associated with serious adverse events, such as cardiotoxicity, major bleeding, visceral perforations, and thromboembolic disease. In addition, frequent hematologic toxicities, such as anemia, are often observed in these patients and have a potential impact on patients' quality of life. Epoetins are approved for the treatment of anemia in combination with chemotherapy in patients with nonmyeloid malignancies, but they have not been assessed for the treatment of anemia related to biological agent therapies.
Molecularly targeted agents were first used in cancer therapy a few years ago, and their usage has increased over time. However, the small sample sizes of various clinical trials and the combination with other agents (e.g., chemotherapy) have made it difficult to determine the prevalence rates and the patterns of drug-induced anemia and cancer-related fatigue (a major consequence of anemia) over time or to compare them across early-phase trials. Decreased levels of hematocrit [2] and hemoglobin [3] are likely associated with cancer-related fatigue. In one study, the degree of anemia (mild, moderate, or severe) was predictive of fatigue severity (p < .001) [3]. The guidelines for cancer-related fatigue from the National Comprehensive Cancer Network identify anemia as one of the seven common contributing and treatable factors that may potentially reduce fatigue if treated. Grade 3–4 fatigue requires treatment interruption or dose adjustment [4].
In patients with cancer, multiple factors may contribute to the development of anemia, including the malignancy itself, chemotherapy, underlying comorbidities, and blood loss. All targeted agents may cause anemia, but the most common cause of anemia remains chronic disease. However, the hypothetical etiopathogenetic role of targeted agents has not yet been clearly assessed.
Theoretically, the causes of anemia in patients with cancer who are undergoing treatment with biological agents can be grouped into three main categories as reported in the literature: blood loss (related to major or minor bleeding) [5–15], reduced/impaired erythrocyte production [16–21], and increased destruction or reduced survival of RBCs [22–28].
The aim of this review and meta-analysis was to identify the incidence of and the relative risk for anemia in large randomized trials of targeted therapies currently approved for the treatment of solid tumors.
Patients and Methods
Data Source
The search was limited to phase II and III randomized controlled trials (RCTs) and was restricted only to approved, targeted agents in the U.S. or Europe. We searched PubMed for published articles in English with no date restriction (last search performed on December 16, 2011) using the keywords “cetuximab,” “panitumumab,” “trastuzumab,” “lapatinib,” “sunitinib,” “sorafenib,” “everolimus,” “temsirolimus,” “pazopanib,” “imatinib,” “bevacizumab,” “gefitinib,” and “erlotinib” in RCTs.
Inclusion criteria were as follows: (a) comparison of labeled targeted therapy plus best supportive care or placebo or cytokines versus best supportive care or placebo alone or cytokines alone, (b) targeted therapy plus chemotherapy versus chemotherapy alone, (c) patients with solid tumors treated with systemic therapy alone, and (D) RCTs. Selected studies were excluded if they included radiotherapy or different targeted drugs in both arms, but they were included if the experimental arm tested two targeted therapies and the control arm tested one targeted agent that was the same as the experimental arm (e.g., [chemotherapy and/or placebo or best supportive care] + A + B versus [chemotherapy and/or placebo or best supportive care] + A, where A and B are both biological agents).
Study Selection
The goal of this study was to determine whether or not targeted therapies contribute to the development of anemia in patients with solid cancer (hematologic malignancies were excluded). We only selected RCTs in which patients treated with and without these agents had been directly compared. Phase I and single-arm phase II trials were excluded because of the lack of a control group. In particular, clinical trials that met the following criteria were included in the meta-analysis: (a) prospective phase II and III randomized, clinical trials in patients with solid tumors; (b) random assignment to either targeted-agent treatment and best supportive care versus best supportive care (or placebo) alone or targeted agents plus concurrent chemotherapy or hormonal agents or biologic response modifiers (e.g., cytokines) versus chemotherapy or hormonal agents or biologic response modifiers alone; and (c) available data for the analysis, including events or incidence of anemia and sample size.
Statistical Endpoints
Details about the number of patients, the type of cancer, the type of treatment, the results, and the follow-up were extracted from the included studies [29]. Data regarding the occurrence of anemia were obtained from the safety profile of each study and were primary endpoints. The cases of anemia in these studies were assessed and recorded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE), which are widely used in cancer clinical trials [30, 31]. Anemia is a diagnosis (not a CTCAE term per se) that results from a reduction in the number of circulating erythrocytes or in the quantity of hemoglobin.
Grade 1 or 2 adverse events were defined as hemoglobin levels between the normal limit and 10 g/dL and 8–10 g/dL, respectively. Grade 3 and 4 adverse events were defined as hemoglobin levels of 6.5–8 g/dL and <6.5 g/dL, respectively. We calculated the relative risk (RR) for all grades of anemia. Data were included if they were reported according to either severe anemia only (grade 3–4) or all grades (grade 1–4) when available. If the percentage of events alone was reported in the experimental and control arms, then the absolute number of events was calculated. Primary analyses were as follows: (a) RR for all grades of anemia in all studies; (b) all-grade, low-grade, and high-grade anemia with targeted therapies alone; and (c) all-grade, low-grade, and high-grade anemia with targeted therapies combined with chemotherapies or other agents. Secondary analyses included the following: (a) the risk for anemia with each targeted agent, (b) the risk for anemia according to the class of targeted therapy; (c) the risk for anemia according to the type of pharmacological agent (tyrosine kinase inhibitors [TKIs] versus monoclonal antibodies); and (d) the risk for anemia according to underlying disease.
Statistical Analysis
RevMan 5.0.24 (Cochrane Information Management System, San Francisco, CA) was used for the statistical analyses. To calculate the RR, patients assigned to the experimental group were only compared with patients assigned to the control group in the same clinical trial. For the meta-analysis, we used either a fixed effects model (weighted with inverse variance) or a random effects model [32]. For each meta-analysis, Cochran's Q statistic and I2 statistics were first calculated to assess heterogeneity among the proportions of the included trials. If p < .1, the assumption of homogeneity was deemed invalid and the random effects model was reported after exploring the causes of heterogeneity [33]. Otherwise, the fixed effects model was reported.
The extent to which the combined risk estimate might be affected by the individual studies was assessed by consecutively omitting each study from the meta-analysis (leave-one-out procedure). Subgroup analyses involving more homogeneous studies (all patients enrolled in trials that explored the benefit of one specific drug or all patients affected by the same disease) were performed to identify subsets of patients who were more likely to suffer from anemia. To detect publication biases, a funnel plot was used, in which the asymmetry was formally investigated with the Egger linear regression approach and the Begg rank correlation test. The impact of publication biases on the summary effects was assessed using the trim-and-fill method. Two-tailed p < .05 was considered statistically significant.
Results
The search found 731 publications, of which only 52 matched the inclusion criteria of this search [6, 34–84]. The number of patients available for toxicity analysis was 24,310. The results of the meta-analysis affirmed that anemia is a frequent event in patients with solid tumors treated with biological agents alone or in combination with chemotherapy. The global incidence of anemia was 22.2% (all grades). The incidence of grades 1–2 and 3–4 adverse events were 31.4% (only 28 trials reported these low-grade anemia events) and 6.3% (all trials), respectively. However, these values are probably underestimated because several trials only reported severe anemia (grade 3–4) and not the more frequent low grades.
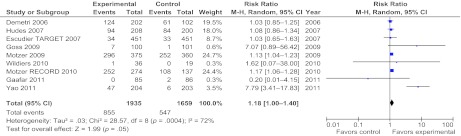
Primary analysis showed that the RR in all the included studies for all grades of anemia was 1.07 (p = .09) (Fig. 1) with high heterogeneity among trials (χ2, 188.13; df, 51; p < .00001; I2, 73%). The corresponding RR was 1.18 (95% confidence interval [CI], 1–1.4), which was significant (p = .05 according to a random effects model) for biologic single-agent trials only (Fig. 2).
Figure 1.
Meta-analysis of the overall relative risk for anemia (all grades) with targeted therapies (all agents separately) in 53 randomized studies.
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel.
Figure 2.
Meta-analysis of the overall relative risk for anemia (all grades) with targeted therapies alone (single-agent trials) versus control arm care in nine randomized studies.
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel.
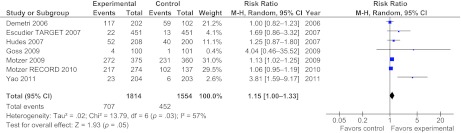
The RRs for low-grade anemia (grade 1–2) were 1.13 (p = .09 according to a random effects model) and 1.15 (95% CI, 1–1.33, p = .05 according to a random effects model) (Fig. 3) for all trials pooled together and biologic agents alone, respectively. The risk ratio calculation of high-grade anemia (grade 3–4) was not significant. However, the results of the test for subgroup differences were highly significant (p < .00001), so it was more reasonable to analyze the RRs for different agents and classes of drugs (Fig. 1).
Figure 3.
Meta-analysis of the overall relative risk for grade 1–2 anemia with targeted therapies alone (single-agent trials) versus control arm care in seven randomized studies.
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel.
Subgroup Analysis
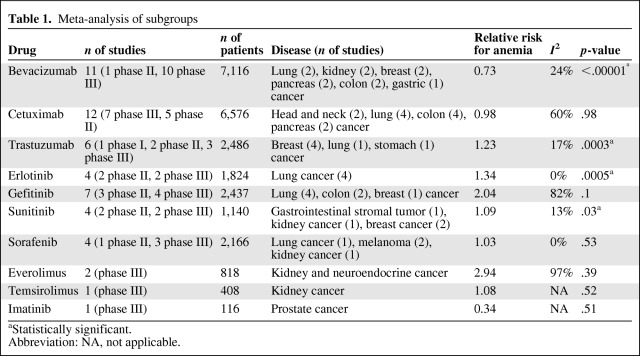
We performed multiple analyses as a function of any biological agent and of any class of agent used, including anti–human epidermal growth factor receptor (HER)-2, anti–epidermal growth factor receptor (EGFR), anti–vascular endothelial growth factor (VEGF), and mammalian target of rapamycin (mTOR) inhibitors. I2 statistics were calculated to assess heterogeneity, and the Z test was used to assess the overall effect (Tables 1 and 2).
Table 1.
Meta-analysis of subgroups
aStatistically significant.
Abbreviation: NA, not applicable.
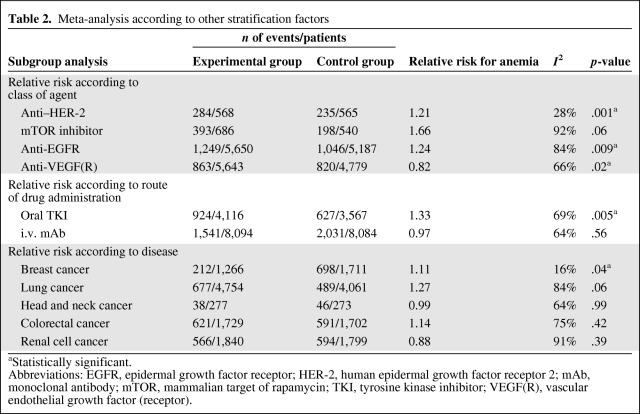
Table 2.
Meta-analysis according to other stratification factors
aStatistically significant.
Abbreviations: EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor 2; mAb, monoclonal antibody; mTOR, mammalian target of rapamycin; TKI, tyrosine kinase inhibitor; VEGF(R), vascular endothelial growth factor (receptor).
Erlotinib, an anti-EGFR TKI approved for the treatment of lung and pancreatic cancer, was associated with a relatively high incidence of anemia (25%) and had an RR of 1.34 (95% CI, 1.14–1.58; p = .0005 according to a fixed effects model) (Fig. 1). These results were significant and similar to the ones obtained with the TKI gefitinib, for which the incidence of anemia was lower (13%) and the RR was 2.04 (95% CI, 0.88–4.76; p = .1 according to a random effects model) (Fig. 1). In particular, a higher RR was observed in patients with lung cancer treated with erlotinib or gefitinib (RR, 1.98; 95% CI, 1.22–3.22; p = .006 according to a random effects model), which resulted in an absolute risk difference of 7%.
Cetuximab, an anti-EGFR monoclonal antibody, was associated with a high risk for anemia (25%), but no higher than in control arms when added to chemotherapy (RR, 0.98; 95% CI, 0.88–1.11; p = 0.8 according to a random effects model) (Fig. 1). Bevacizumab, which is an anti-VEGF monoclonal antibody used for treating breast, lung, kidney, and colorectal carcinoma, was associated with a lower risk for anemia than in nonbevacizumab arms (RR, 0.73; p < .00001 according to a fixed effects model, absolute risk difference, −3%), with similar results across all pathologies (Fig. 1).
In regard to the other anti-VEGFR agents (sunitinib and sorafenib), the risk for anemia was significantly higher only in the sunitinib arms (RR, 1.09; 95% CI, 1.01–1.18; p = .03 according to a fixed effects model) (Fig. 1). When we only considered monotherapy trials (sunitinib and sorafenib studies), the respective incidence values were 78% and 7.5%. The value for sunitinib was most likely higher because most patients were affected by renal cell carcinomas, for which anemia is a very frequent disease-related laboratory event (74% of the analysis was taken from a study by Motzer et al. [59]; RR, 1.13; p = .006). This is not true for the Escudier et al. [38] trial that was conducted in patients with renal cell carcinomas treated with sorafenib, in which the RR of 1.03 was not found to be significant. The pooled analysis of all trials, including the monotherapy arms only (sunitinib or sorafenib), showed an RR of 1.1 (p = .03; absolute risk difference, 4%).
In the studies that compared arms using the anti–HER-2 monoclonal antibody trastuzumab with nontrastuzumab arms (combination arms only), the incidence of anemia was 42% in the experimental arms, with a higher risk than in the control arms (RR, 1.23; 95% CI, 1.10–1.37; p = .0003 according to a fixed effects model; absolute risk difference, 8%) (Fig. 1).
The incidences of anemia reported in the two studies with mTOR inhibitors (everolimus and temsirolimus) were the highest reported in our analysis (62.5% and 53%, respectively); the RRs were 1.43 (p <.00001) and 1.08 (p = .52), respectively (Fig. 1). We also analyzed the RR for developing anemia according to the type and class of agent used. In particular, TKIs were found to be associated with an RR of 1.33 (95% CI, 1.09–1.62; p = .005 according to a random effects model), whereas monoclonal antibodies were associated with a lower RR for anemia (RR, 0.97; p = .56).
Anti-EGFR, anti–HER-2, anti-VEGFR, and mTOR inhibitors predicted RRs of 1.24, 1.20, 0.82, and 1.66, respectively; all these values were significant (p = .009, p = .0003, p = .02, and p = .06, respectively). According to the pathology-driven analysis, patients with colorectal cancer, breast cancer, head-neck cancer, renal cell carcinoma, and non-small cell lung cancer (NSCLC) did not exhibit any significantly different risks for developing anemia if treated with targeted therapies, with the exception of breast cancer (RR, 1.11; p = .04). The results are summarized in Table 1 according to the different agents used.
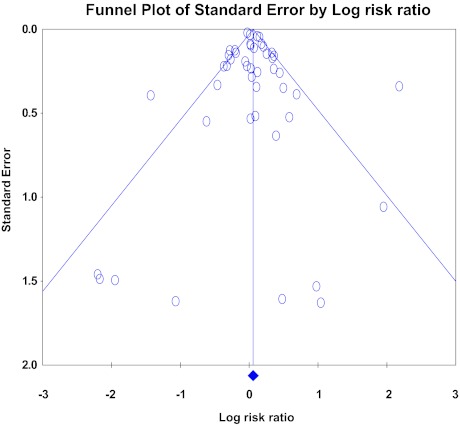
The absence of a dominant study driving the results of the meta-analysis was demonstrated by the “one-study-removed” procedure that generated overall risk ratio estimates (RR, 1.06; range, 0.92–1.44; p = .132). We also investigated publication biases, which were not statistically significant (p = .46981, Begg and Mazumdar rank correlation test; p = .28742, Egger regression test). Consequently, we calculated the number of potentially “missing” trials according to the trim-and-fill method mentioned above, which suggested that two studies were missing; however, according to the random effects model, the RR estimate was 1.03 (0.947–1.121). The funnel plots are represented in Figure 4.
Figure 4.
Funnel plot of the meta-analysis.
Discussion
Anemia is a frequent but often underreported and undertreated [85] event in clinical practice. The treatment of anemia is also expensive for the health care system in terms of blood transfusions, erythropoiesis-stimulating agents (ESAs), and parenteral iron. Anemia is associated with a poor prognosis in patients with cancer and was shown to be correlated with a 65% greater overall mortality risk [86, 87]. In the largest trial that was analyzed in this study (the Erbitux Plus Irinotecan in Colorectal Cancer trial) [68], anemia was the most frequently occurring adverse event among hematological and nonhematological adverse events, affecting 85% and 87% of patients in each arm, respectively. In addition, some degree of anemia could cause fatigue, which is a common event associated with these agents. The risk for manifesting fatigue was 36% higher than in control arms with a risk difference of 10% in the trials involving targeted drugs as single agents, in which the RR for anemia was 1.18. However, after adjusting for anemia rate (with metaregression analysis), the risk for fatigue remained significantly higher than in control arms. Therefore, fatigue is an additional side effect of certain biological agents, linked to but independent from anemia.
Compared with standard therapy (chemotherapy or supportive care), our analysis showed that the overall RR for targeted agent–related anemia was 1.07 (incidence, 22%), whereas this value was significantly higher for erlotinib (incidence, 25%) and gefitinib (incidence, 13%; RR, 1.34 and 2.04, respectively). One explanation for this result is that the four analyzed erlotinib studies enrolled patients with advanced NSCLC who were also being treated (except for one) with platinum-based chemotherapy combined with erlotinib. In general, the RR was higher for targeted therapies alone (monotherapy studies) than in combination with other antineoplastic agents (RR, 1.18). Similarly, the RR for anemia was significantly higher for mTOR inhibitors, anti-EGFR or anti–HER-2 agents, sunitinib, and sorafenib (incidence, 66%) and lower for the anti-VEGF monoclonal antibody bevacizumab (RR, 0.73).
The mechanism of action of bevacizumab is only mediated by circulating VEGF blockade. In contrast, TKIs, such as sunitinib, instead target several receptor tyrosine kinases in tumors and endothelial cells. These pathways most likely play critical roles in the development of anemia. One explanation for anemia with multitargeted TKIs is the action on hematopoiesis of FLT-3 and Kit blockade [20, 88, 89]. This probably explains some forms of macrocytosis seen during sunitinib therapy and mentioned previously [16–20]. Chronic bleeding resulting from these angiogenetic drugs cannot be ruled out [90]. Also, some cases of microangiopathic thrombotic hemolytic anemia were observed by some authors, particularly with sunitinib [22–28, 58].
A previous meta-analysis of bevacizumab RCTs is in agreement with our results, showing a risk-lowering effect of bevacizumab on anemia adverse events [5, 91, 92]. The only monoclonal antibody that seemed to increase the risk for anemia was trastuzumab (RR, 1.23). Overall, the risk was higher with orally available TKIs than with monoclonal antibodies (RR, 1.33 versus 0.96, respectively), which reinforces the role of these orally available agents in the etiopathogenesis of anemia.
The mildly higher incidence of low-grade anemia, however, does not seem to be associated with a detrimental effect on quality-of-life parameters, with the exception of fatigue, which is a very common side effect of targeted therapies. Only 20% of the analyzed studies reported data regarding quality of life. Nevertheless, the effect of treatment on cancer-related symptoms exceeded the effect of anemia.
The results from this meta-analysis are only partially explainable. There is most likely an association between targeted agents (e.g., erlotinib and gefitinib) and the type of cancer. For example, in patients with lung cancer treated with anti-EGFR TKIs, the RR for anemia was more than double that of patients treated in control arms (RR, 1.81; p = 0.006 for the overall effect in this subgroup). Lung cancer is frequently associated with anemia, and platinum-based therapies frequently cause anemia and are critical components of this type of treatment approach. Similarly, renal cell carcinoma is often associated with disease- or paraneoplastic-related anemia. Targeted agents approved for the treatment of renal cell carcinoma (e.g., sunitinib) increase the RR for anemia (RR, 1.1) and are frequently associated with anemic adverse events (66% in all studies reported). In our analysis, however, only NSCLCs (not renal cell carcinomas) were associated with a higher RR for anemia per se when treated with biological agents (RR, 1.27 versus 0.88 for the two comparisons, respectively), even though both results were not significant. This result seems to support the independent role of these drugs as causative agents of anemia in addition to the underlying disease.
The meta-analysis showed that anemia associated with targeted therapy alone is a frequent event in clinical practice and that treatment of anemia remains a challenge. In particular, grade 1–2 anemia is more frequent than grade 3–4 anemia, which usually requires a blood transfusion. No approved treatment is currently available for mild anemia caused by these drugs, and iron supplementation, correction of other additional causes of anemia, reduction or interruption of the treatment, and transfusion are the only available approaches to prevent or treat this condition. According to the major international guidelines for anemia, ESAs are to only be used in patients with chemotherapy-induced anemia [93–96]. Because of the high incidence of targeted agent–related anemia, the frequent coexistence of drug-related fatigue, and the prolonged chronic treatments that patients undergo, the use of epoetins as antianemic therapy could provide an opportunity for evaluation with ad hoc studies.
There is an urgent need to perform additional studies that further clarify the real pathogenetic mechanism of targeted agents in the development of anemia. In addition, the extension of ESA labels in this setting can only be considered after careful evaluation through appropriate, well-conducted RCTs. A clear understanding of these issues is crucial for clinicians to inform patients about the potential benefits and harmful effects of these drugs, as well as to extend the benefit and reduce the related risks of these treatments.
Conclusion
The meta-analysis described here has shown that the incidence of anemia is a considerable event in 52 published RCTs with targeted therapies, with a particularly significant incidence of grade 1–2 anemia adverse events (31%). The overall RR compared with patients treated with nontargeted therapies was 1.07 but was not significant. The RRs for all grades as well as for grade 1–2 anemia in patients treated with targeted agents as monotherapy were significant, compared with supportive care alone. TKIs and mTOR inhibitors (particularly erlotinib, gefitinib, sunitinib, everolimus, and temsirolimus) were found to be associated with higher and significant RRs. Among monoclonal antibodies, trastuzumab was the only agent with a significant association, whereas bevacizumab was associated with a lower risk for anemia. The RR was higher for oral TKI agents than for parenteral monoclonal antibodies, particularly in the setting of advanced lung cancer. The biological explanation for these data is unknown, but an association with the underlying disease (e.g., lung carcinoma), stage of disease (e.g., metastatic), and paraneoplastic syndromes may play an important role. In particular, the use of biological agents alone increased the risk for grade 1–2 anemia by 15%.
It is yet unknown if the label of ESA agents could be extended to patients treated with targeted therapies. However, the risk-to-benefit ratio of ESA agents in this population has to be carefully explored with appropriate clinical trials, particularly with regard to vascular adverse events.
Author Contributions
Conception/Design: Sandro Barni, Mary Cabiddu, Fausto Petrelli
Provision of study material or patients: Sandro Barni, Mary Cabiddu, Paolo Guarneri, Veronica Lonati, Fausto Petrelli
Collection and/or assembly of data: Sandro Barni, Mary Cabiddu, Paolo Guarneri, Veronica Lonati, Fausto Petrelli
Data analysis and interpretation: Sandro Barni, Mary Cabiddu, Veronica Lonati, Fausto Petrelli
Manuscript writing: Sandro Barni, Mary Cabiddu, Paolo Guarneri, Fausto Petrelli
Final approval of manuscript: Sandro Barni, Mary Cabiddu, Paolo Guarneri, Veronica Lonati, Fausto Petrelli
References
- 1.Hurter B, Bush NJ. Cancer-related anemia: Clinical review and management update. Clin J Oncol Nurs. 2007;11:349–359. doi: 10.1188/07.CJON.349-359. [DOI] [PubMed] [Google Scholar]
- 2.Payne J, Piper B, Rabinowitz I, et al. Biomarkers, fatigue, sleep, and depressive symptoms in women with breast cancer: A pilot study. Oncol Nurs Forum. 2006;33:775–783. doi: 10.1188/06.ONF.775-783. [DOI] [PubMed] [Google Scholar]
- 3.Cella D, Lai JS, Chang CH, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology. [accessed December 16, 2011]. Available at http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- 5.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Phase III trial of Avastin plus chemotherapy showed 30 percent improvement in overall survival in first-line non-squamous, non-small cell lung cancer. [accessed April 3, 2012]. Available at http://www.roche.com/inv-update-2005–05-16.
- 8.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advance non-small-cell lung cancer. J Clin Oncol. 2008;26:650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 12.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 13.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 14.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: Safety profile and management of adverse events. Semin Oncol. 2006;33:S26–S34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 16.Gkountouvas A, Kostoglou-Athanassiou I, Veniou E, et al. Hematologic toxicity in patients treated with sunitinib for advanced thyroid cancer. Thyroid. 2010;20:597–600. doi: 10.1089/thy.2010.0028. [DOI] [PubMed] [Google Scholar]
- 17.Price J, Shaarbaf R, Wood L. Sunitinib causes macrocytosis in patients with advanced renal cell carcinoma. Curr Oncol. 2010;17:30–33. doi: 10.3747/co.v17i2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schallier D, Trullemans F, Fontaine C, et al. Tyrosine kinase inhibitor-induced macrocytosis. Anticancer Res. 2009;29:5225–5228. [PubMed] [Google Scholar]
- 19.Billemont B, Izzedine H, Rixe O. Macrocytosis due to treatment with sunitinib. N Engl J Med. 2007;357:1351–1352. doi: 10.1056/NEJMc071867. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Choueiri TK, Elson P, et al. Sunitinib-induced macrocytosis in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1309–1314. doi: 10.1002/cncr.23711. [DOI] [PubMed] [Google Scholar]
- 21.Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 22.Bevacizumab + sunitinib: Microangiopathic haemolytic anaemia. A serious drug interaction between 2 cancer drugs. Prescrire Int. 2009;18:165. [PubMed] [Google Scholar]
- 23.Jain R, Mathew P, Wood CG, Tannir NM. Sunitinib-induced acute hemolysis without hypertension: A case report. Clin Genitourin Cancer. 2008;6:122–123. doi: 10.3816/CGC.2008.n.019. [DOI] [PubMed] [Google Scholar]
- 24.Berchem G, Dewilde S, Mahassen P. A case of acute haemolysis with 2 different multi target thyrosine kinase inhibitors in a patient with renal cancer. Bull Soc Sci Med Grand Duche Luxemb. 2009;1:7–9. [PubMed] [Google Scholar]
- 25.Kapiteijn E, Brand A, Kroep J, et al. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007;18:1745–1747. doi: 10.1093/annonc/mdm454. [DOI] [PubMed] [Google Scholar]
- 26.Siau K, Varughese M. Thrombotic microangiopathy following docetaxel and trastuzumab chemotherapy: A case report. Med Oncol. 2010;27:1057–1059. doi: 10.1007/s12032-009-9333-6. [DOI] [PubMed] [Google Scholar]
- 27.Frangié C, Lefaucheur C, Medioni J, et al. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 2007;8:177–178. doi: 10.1016/S1470-2045(07)70037-2. [DOI] [PubMed] [Google Scholar]
- 28.Bollée G, Patey N, Cazajous G, et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant. 2009;24:682–685. doi: 10.1093/ndt/gfn657. [DOI] [PubMed] [Google Scholar]
- 29.Meade MO, Richardson WS. Selecting and appraising studies for a systematic review. Ann Intern Med. 1997;127:531–537. doi: 10.7326/0003-4819-127-7-199710010-00005. [DOI] [PubMed] [Google Scholar]
- 30.NCI Common Terminology Criteria for Adverse Events. [accessed December 16, 2011]. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 31.NCI Common Terminology Criteria for Adverse Events. [accessed December 16, 2011]. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev2to3.pdf.
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 34.Butts CA, Bodkin D, Middleman EL, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin, with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J Clin Oncol. 2007;25:5777–5784. doi: 10.1200/JCO.2007.13.0856. [DOI] [PubMed] [Google Scholar]
- 35.Burtness B, Goldwasser MA, Flood W, et al. Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 36.Cascinu S, Berardi R, Labianca R, et al. Italian Group for the Study of Digestive Tract Cancer (GISCAD). Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: A randomised, multicentre, phase II trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]
- 37.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 38.Escudier B, Eisen T, Stadler WM, et al. TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 39.Escudier B, Pluzanska A, Koralewski P, et al. AVOREN Trial investigators. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 40.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 41.Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. 2004;15:19–27. doi: 10.1093/annonc/mdh031. [DOI] [PubMed] [Google Scholar]
- 42.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial–INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol. 2009;27:2253–2260. doi: 10.1200/JCO.2008.18.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guarneri V, Frassoldati A, Ficarra G, et al. Phase II, randomized trial of preoperative epirubicin-paclitaxel +/- gefitinib with biomarker evaluation in operable breast cancer. Breast Cancer Res Treat. 2008;110:127–134. doi: 10.1007/s10549-007-9688-3. [DOI] [PubMed] [Google Scholar]
- 45.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 46.Herbst RS, Prager D, Hermann R, et al. TRIBUTE Investigator Group. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 47.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial–INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 48.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 49.Hudes G, Carducci M, Tomczak P, et al. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 50.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. FinHer Study Investigators. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 51.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: Results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28:911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 52.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 53.Mathew P, Thall PF, Bucana CD, et al. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–5824. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 54.Mayer EL, Dhakil S, Patel T, et al. SABRE-B: An evaluation of paclitaxel and bevacizumab with or without sunitinib as first-line treatment of metastatic breast cancer. Ann Oncol. 2010;21:2370–2376. doi: 10.1093/annonc/mdq260. [DOI] [PubMed] [Google Scholar]
- 55.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: A report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–2185. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 56.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 57.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 58.Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:5080–5087. doi: 10.1200/JCO.2008.21.5541. [DOI] [PubMed] [Google Scholar]
- 59.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motzer RJ, Escudier B, Oudard S, et al. RECORD-1 Study Group. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 61.Pirker R, Pereira JR, Szczesna A, et al. FLEX Study Team. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 62.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 63.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol. 2008;19:362–369. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 65.Santoro A, Comandone A, Rimassa L, et al. A phase II randomized multicenter trial of gefitinib plus FOLFIRI and FOLFIRI alone in patients with metastatic colorectal cancer. Ann Oncol. 2008;19:1888–1893. doi: 10.1093/annonc/mdn401. [DOI] [PubMed] [Google Scholar]
- 66.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 67.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 68.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 69.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 70.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 71.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 72.Borner M, Koeberle D, Von Moos R, et al. Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: A randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol. 2008;19:1288–1292. doi: 10.1093/annonc/mdn058. [DOI] [PubMed] [Google Scholar]
- 73.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 74.Wildiers H, Fontaine C, Vuylsteke P, et al. Multicenter phase II randomized trial evaluating antiangiogenic therapy with sunitinib as consolidation after objective response to taxane chemotherapy in women with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2010;123:463–469. doi: 10.1007/s10549-010-1066-x. [DOI] [PubMed] [Google Scholar]
- 75.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bang YJ, Van Cutsem E, Feyereislova A, et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomized controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 78.Stathopoulos GP, Batziou C, Trafalis D, et al. Treatment of colorectal cancer with and without bevacizumab: A phase III study. Oncology. 2010;78:376–381. doi: 10.1159/000320520. [DOI] [PubMed] [Google Scholar]
- 79.Gaafar RM, Surmont VF, Scagliotti GV, et al. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03) Eur J Cancer. 2011;47:2331–2340. doi: 10.1016/j.ejca.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 80.Viéitez JM, Valladares M, Peláez I, et al. A randomized phase II study of raltitrexed and gefitinib versus raltitrexed alone as second line chemotherapy in patients with colorectal cancer. (1839IL/0143) Invest New Drugs. 2011;29:1038–1044. doi: 10.1007/s10637-010-9400-z. [DOI] [PubMed] [Google Scholar]
- 81.Stinchcombe TE, Peterman AH, Lee CB, et al. A randomized phase II trial of first-line treatment with gemcitabine, erlotinib, or gemcitabine and erlotinib in elderly patients (age ≥70 years) with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. 2011;6:1569–1577. doi: 10.1097/JTO.0b013e3182210430. [DOI] [PubMed] [Google Scholar]
- 82.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 85.Verbeke N, Beguin Y, Wildiers H, et al. High prevalence of anaemia and limited use of therapy in cancer patients: A Belgian survey (Anaemia Day 2008) Support Care Cancer. 2012;20:23–28. doi: 10.1007/s00520-010-1045-0. [DOI] [PubMed] [Google Scholar]
- 86.Harper P, Littlewood T. Anaemia of cancer: Impact on patient fatigue and long-term outcome. Oncology. 2005;69(suppl 2):2–7. doi: 10.1159/000088282. [DOI] [PubMed] [Google Scholar]
- 87.Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 88.Weisel KC, Yildirim S, Schweikle E, et al. Effect of FLT3 inhibition on normal hematopoietic progenitor cells. Ann N Y Acad Sci. 2007;1106:190–196. doi: 10.1196/annals.1392.020. [DOI] [PubMed] [Google Scholar]
- 89.Kimura Y, Ding B, Imai N, et al. c-Kit-mediated functional positioning of stem cells to their niches is essential for maintenance and regeneration of adult hematopoiesis. PLoS One. 2011;6:e26918. doi: 10.1371/journal.pone.0026918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: A sys-tematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 91.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 92.Sher AF. Effect of bevacizumab on the risk of chemotherapy-associated anemia in cancer patients: A meta-analysis. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 93.NCCN Clinical Practice Guidelines in Oncology. [accessed December 16, 2011]. Available at http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf.
- 94.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology; American Society of Hematology. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28:4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- 95.Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. The Oncologist. 2008;13(suppl 3):33–36. doi: 10.1634/theoncologist.13-S3-33. [DOI] [PubMed] [Google Scholar]