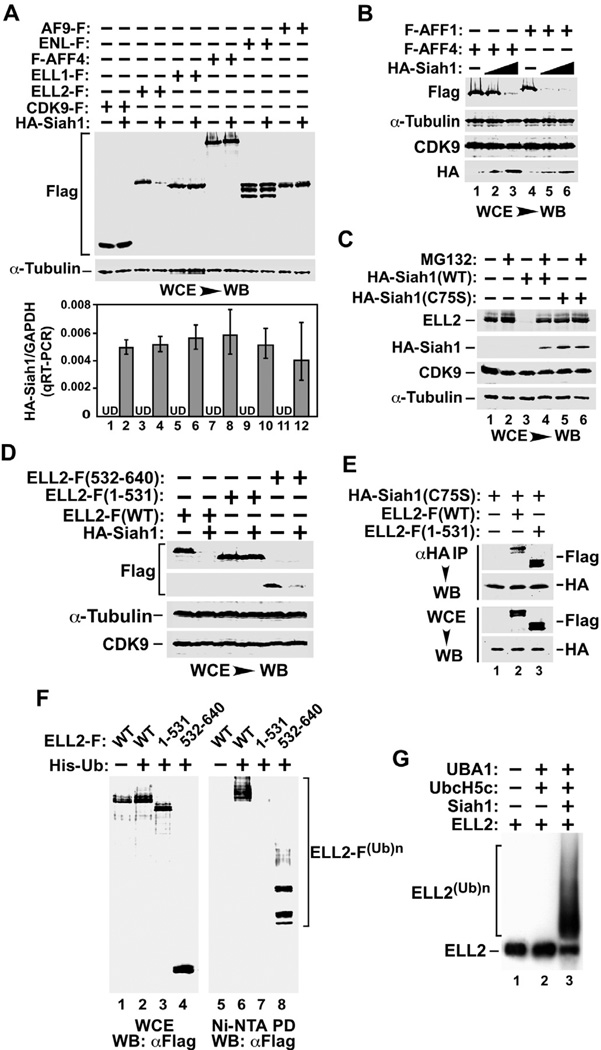
Figure 3. ELL2 is more susceptible than AFF1 and AFF4 to degradation induced by Siah1, an E3 ubiquitin ligase that directly polyubiquitinates the ELL2 C-terminal region in vivo and in vitro.
A. & B. HeLa cells were transfected with (+) or without (−) the indicated expression constructs and whole cell lysates (WCE) were examined by Western blotting (WB) with the indicated antibodies. The HA-Siah1 mRNA levels produced from the transfected plasmid were determined by qRT-PCR and the ratios over endogenous GAPDH mRNA were shown in the bottom panel of A. The error bars represent mean +/− SD from three independent experiments. UD: undetectable. The HA-Siah1 construct was transfected in 3-fold increment in B. C. HeLa cells were transfected with plasmids expressing either WT or the C75S mutant HA-Siah1 and then treated with (+) or without (−) MG132. WCE were examined by WB for the indicated proteins. D. WCE of transfected cells were analyzed by WB as in A. E. WCE of HeLa cells transfected with the indicated expression constructs and anti-HA IP derived from WCE were analyzed by anti-HA and –Flag Western blotting. F. HeLa cells were transfected with constructs expressing WT or truncated ELL2-F and His-Ub as indicated. WCE as well as polyubiquitinated ELL2 isolated from WCE by Ni2+-NTA pull down (PD) were analyzed by anti-Flag WB as in Fig. 1A. G. In vitro ubiquitination reactions containing the indicated components were performed and 35S-labeled WT ELL2 was detected by autoradiography.