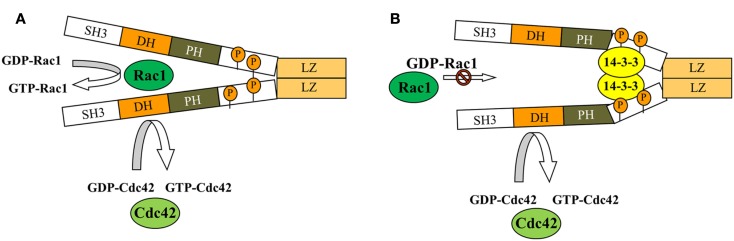
Figure 4.
Model depicting how binding of 14-3-3 inhibits dimeric β1Pix-GEF activity. Model depicting how binding of 14-3-3β inhibits dimeric β1Pix-GEF activity. (A) Under basal conditions, the binding of 14-3-3β to β1Pix is minimal and does not affect its GEF activity. (B) In the presence of agonist, binding of 14-3-3β to β1Pix through S516 and T526 is increased. 14-3-3β binding may either block the interaction between Rac1 and the DH domain of β1Pix or induce a conformational change of the DH domain that would interfere with GTP binding (modified from Chahdi and Sorokin, 2008b with permission). As shown, Cdc42 activity could be regulated by monomeric β1Pix. 14-3-3β is unable to bind monomeric β1Pix, while binding of 14-3-3β to dimeric β1Pix results in inhibition of its GEF activity toward Rac1.