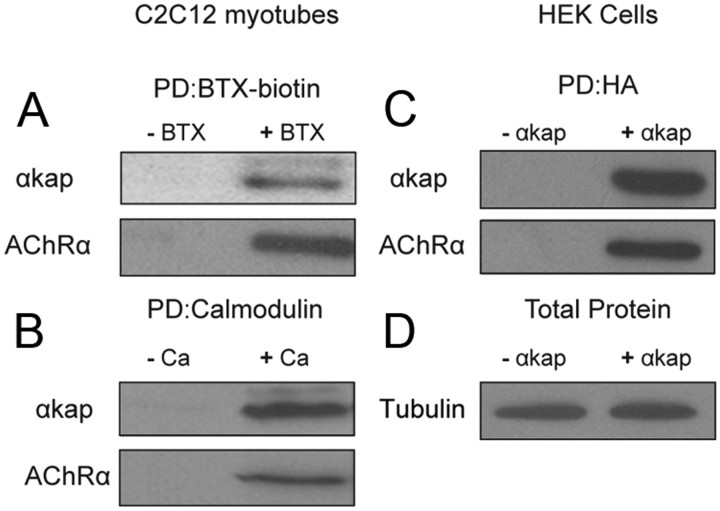
Figure 1.
αkap forms complexes with AChR in skeletal muscle cells. A, Lysates from cultured C2C12 myotubes were incubated with (+ BTX) or without (− BTX) BTX–biotin, and then biotin containing complexes were isolated with NeutrAvidin beads. Pull-down proteins (PD) were probed with anti-αkap or anti-AChRα antibody. B, Myotube lysates were incubated with calmodulin beads in the absence of calcium (− Ca) or in the presence 2 mm calcium (+ Ca) to pull down calcium/calmodulin binding proteins. Blots were probed with anti-αkap or anti-AChRα antibody. C, Lysates from human embryonic kidney (HEK293T) cells transfected with AChRα and αkap–HA (+ αkap) or with AChRα and empty plasmid (− αkap) were immunoprecipitated with anti-HA-coupled agarose beads, and blots were probed with anti-αkap or anti-AChRα antibodies. D, Aliquots from the lysates used for the pull downs in C probed for tubulin. For all four panels, at least three independent experiments were performed.