Abstract
Background
Uncertainties persist concerning the effects of early intensive management of type 2 diabetes and which patients benefit most from such an approach.
Aim
To describe change in modelled cardiovascular risk in the 14 months following diagnosis, and to examine which baseline patient characteristics and treatment components are associated with risk reduction.
Design and setting
A cohort of individuals from a pragmatic, single-blind, cluster-randomised controlled trial of 236 females and 361 males with screen-detected type 2 diabetes and without prior cardiovascular disease (CVD), from 49 GP surgeries in eastern England, examined at baseline (2002–2006) and after 14-months’ follow-up.
Method
Multiple linear regression was used to quantify the association between baseline patient characteristics, treatment components, and change in modelled 10-year cardiovascular risk (UK Prospective Diabetes Study [UKPDS] [version 3] risk engine).
Results
There was a downward shift in the distribution of modelled CVD risk over 14 months mean 31% (standard deviation [SD] = 14%) to 26% [SD = 13%]). Older individuals, males, and those with a larger waist circumference at baseline exhibited smaller risk reductions. Individuals prescribed higher numbers of drugs over the follow-up period, and those who decreased their energy intake or reduced their weight, demonstrated larger reductions in modelled risk.
Conclusion
It is possible to achieve significant reductions in modelled CVD risk over 14 months following diagnosis of diabetes by screening. Risk reduction appeared to be driven mainly by prescription of higher numbers of drugs, decreased energy intake, and weight reduction. There was room for further risk reduction, as many patients were not prescribed recommended treatments.
Keywords: cardiovascular diseases, prevention and control; diabetes mellitus; equity; mass screening; primary care; risk factors; socioeconomic factors
INTRODUCTION
Population-based screening and early treatment for type 2 diabetes could potentially reduce the growing public health burden of this disease.1,2 Several trials suggest that intensive treatment of individual risk factors is effective in those with clinically diagnosed type 2 diabetes.3–6 However, there is little evidence to inform the treatment of screen-detected patients. One-year results from the Dutch centre of the ADDITION-Europe trial (Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen Detected Diabetes in Primary Care),7 showed that intensive multifactorial treatment of screen-detected individuals was associated with significant reduction in cardiovascular risk factors without worsening quality of life.8 Nonetheless, uncertainties persist concerning the potential magnitude of risk reduction achievable in the first year after diagnosis, and the factors associated with reduction in risk.
Research suggests that practitioners might be reluctant to recommend intensive treatment to asymptomatic individuals with screen-detected diabetes.9 Furthermore, individuals at high risk of diabetes exhibit characteristics that have previously been associated with inequality in healthcare provision, including low socioeconomic status,10 black and minority ethnic status,11 older age,12 and obesity.13,14 Social inequalities exist in the treatment of patients with coronary heart disease and diabetes.15–19 Evidence of specific inequities in service provision or uptake might inform the content and implementation of treatment policies in patient with early-diagnosed diabetes.
The aims of this analysis are to describe the change in modelled cardiovascular disease (CVD) risk in the first year after diagnosis in a cohort of individuals with screen-detected type 2 diabetes; explore which baseline patient characteristics and which treatment components are associated with reduction in modelled CVD risk in this screen-detected population; and assess potential variation in the provision of treatment early in the course of the disease.
METHOD
Data are presented from the ADDITION-Cambridge trial cohort. ADDITION-Cambridge is a primary-care-based study of screening for type 2 diabetes, followed by a pragmatic open-label cluster randomised controlled trial comparing intensive multifactorial treatment with routine care in patients with screen-detected diabetes. The design and rationale have previously been reported.20
How this fits in
Uncertainties persist concerning the management of diabetes early in the course of the disease. Consequently, practitioners may be reluctant to intensively treat newly-diagnosed patients identified by screening. This study shows that it is possible to achieve significant shifts in the population distribution of modelled cardiovascular disease risk, by using intensive management in the first year after diagnosis. Risk reduction appeared to be mainly driven by prescription of higher numbers of drugs, decreased energy intake, and weight reduction.
Forty-nine general practice surgeries in the eastern region of England (26 in the intensive-treatment group [IT] and 23 in the routine-care group [RC]) recruited patients. Randomisation and screening processes have been described elsewhere.21 World Health Organization criteria were used to diagnose diabetes.22 All patients newly diagnosed with type 2 diabetes in the screening phase were eligible to participate in the treatment study, unless their GP indicated that they had contraindications to proposed study medication. A total of 867 eligible individuals agreed to participate.
Participants detected with type 2 diabetes were subsequently managed according to the treatment regimen to which their practice was allocated: RC or IT. RC followed the current UK national guidelines for diabetes management.23–25 In the IT practices, the intensification of diabetes management was achieved through the addition of a number of features to existing diabetes care.20
The study aimed to educate and support GPs, practice nurses, and participants in target-driven management (using medication and promotion of healthy lifestyles) of hyperglycaemia, blood pressure, and cholesterol, based on the stepwise regimen used in the Steno-2 study.26 Treatment targets and algorithms were based on trial data demonstrating the benefits of intensive treatment of cardiovascular risk factors in people with type 2 diabetes.4,5,26,27 Targets included glycosylated haemoglobin (HbA1c) <7.0%, blood pressure ≤135/85 mmHg, cholesterol <5 mmol/l without ischaemic heart disease or <4.5 mmol/l with ischaemic heart disease, and prescription of aspirin to those treated with antihypertensive medication. Following publication of the Heart Protection Study,28 the treatment algorithm included a recommendation to prescribe a statin to all patients with a cholesterol level ≥3.5 mmol/l.
Measurement and outcomes
Baseline and 1-year health assessments included physiological and anthropometric measures, venesection, and the completion of questionnaires. Anthropometric and clinical measures were undertaken by trained staff, following standard operating procedures. Data-collection methods have been described previously.20 Standardised questionnaires were used to collect information on sociodemographic characteristics (education and social status) and lifestyle habits (smoking status). Data on medication adherence, physical activity, and dietary behaviour were collected using validated self-report questionnaires: the Medication Adherence Report Scale (MARS),29 the EPIC-Norfolk physical activity questionnaire (EPAQ-2),31 and a food-frequency questionnaire,31 respectively. Individuals were considered to be ‘adherent’ to medication if they attained a MARS score >23/25. Information from EPAQ-2 was used to derive total physical activity and types of physical activity (expressed in metabolic equivalent hours per week). Information from the food-frequency questionnaire was used to derive total energy intake (expressed in kcal).
The primary endpoint at 1-year follow-up was the modelled 10-year risk of cardiovascular disease using the UK Prospective Diabetes Study (UKPDS) engine version 3.0. This is a diabetes-specific absolute risk-assessment tool that defines CVD as the first to occur of myocardial infarction, sudden cardiac death, other incident ischaemic heart disease, stroke, and peripheral vascular disease.
Participants with complete data on the UKPDS score variables (sex, ethnicity, smoking status, presence or absence of atrial fibrillation, systolic blood pressure, HbA1c, total cholesterol, high-density lipoprotein (HDL)-cholesterol), and without a self-reported history of macrovascular disease, were assessed. A total 736 participants completed the follow-up health assessment after a mean of 14 months (standard deviation [SD] = 3.0 months); 86 individuals were excluded because they reported a previous CVD event (stroke or myocardial infarction) at follow-up, and 53 people were excluded as they did not have complete data available to calculate the CVD risk score, yielding 597 participants with complete data for analysis.
Statistical analyses
A cohort analysis of ADDITION-Cambridge trial data was conducted. Change in modelled CVD risk was calculated by subtracting baseline CVD risk from follow-up CVD risk. Multiple linear regression models were used to describe the association between baseline patient characteristics, intervention components, and change in CVD risk; results are reported as unstandardised beta coefficients. All models were adjusted for baseline CVD risk and randomisation group, and took clustering by GP practice into account. The residuals of all linear regression models were checked to ensure that they were normally distributed. Models were also run separately by trial arm and by sex. As results were largely similar, the data were pooled and results from linear regression models based on data from the whole cohort are presented. Any difference by trial arm or sex is reported. In the analysis of change in plasma vitamin C, patients who reported taking vitamin C tablets were excluded. Sensitivity analyses were conducted to examine possible differences in 10-year modelled CVD risk in participants with and without a prior CVD event at baseline. All data were analysed using STATA (version 11.0).
RESULTS
On average, modelled 10-year CVD risk declined in the whole cohort over 14 months (Figure 1). At baseline, the mean modelled CVD risk was 31.0% (SD = 14.4%), reducing to 25.7% (SD = 12.6%) at follow-up. The magnitude of the reduction in modelled CVD risk was directly related to baseline CVD risk; participants with the highest modelled risk at baseline demonstrated the biggest decrease in modelled risk, while, on average, individuals with the lowest baseline risk had the smallest change in modelled risk. Baseline patient characteristics are shown in Table 1.
Figure 1.
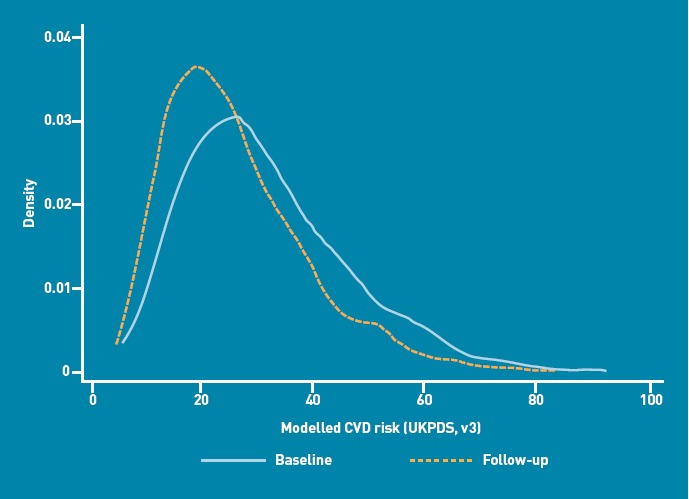
Distribution of 10-year modelled cardiovascular disease (CVD) risk (UKPDS risk engine, version 3) at baseline and follow-up in the ADDITION-Cambridge cohort.
Table 1.
Baseline participant characteristics in the ADDITION-Cambridge cohort, stratified by sex
| Variables | Females (n = 236) | Males (n = 361) |
|---|---|---|
| Mean age, years (SD) | 62.3 (6.1) | 59.8 (7.7) |
| Mean 10-year CVD risk, % (SD) | 22.4 (10.0) | 36.6 (14.1) |
| Ethnicity: white, n (%) | 226 (96) | 353 (98) |
| Post-16 years full-time education, n (%) | 118 (49) | 180 (57) |
| Civil status: married, n (%) | 166 (70) | 294 (70) |
| Current smokers, n (%) | 35 (15) | 64 (18) |
| Median HbA1c, %, (IQR) | 6.7 (6.3–7.6) | 6.9 (6.3–8.0) |
| Mean blood pressure, mmHg (SD) | 139.7 (19.6) | 143.8 (19.4) |
| Systolic | 79.2 (8.9) | 84.2 (10.7) |
| Diastolic | ||
| Mean BMI, kg/m2 (SD) | 34.3 (5.8) | 32.7 (5.2) |
| Mean waist circumference, cm (SD) | 107.3 (13.1) | 114.3 (13.0) |
| Lipids | ||
| Mean total cholesterol, mmol/l (SD) | 5.65 (1.16) | 5.35 (1.03) |
| Median Triglycerides, mmol/l (IQR) | 1.7 (1.2–2.4) | 1.80 (1.3–2.6) |
| Mean HDL-cholesterol, mmol/l (SD) | 1.32 (0.35) | 1.11 (0.30) |
BMI = body mass index. CVD = cardiovascular disease. HDL = high-density lipoprotein. IQR = interquartile range. SD = standard deviation.
Baseline patient predictors of CVD change
Female sex and Asian ethnicity were associated with a significant decrease in 10-year modelled CVD risk, while education, social class, and marital status did not demonstrate any association. Older individuals and those with a larger waist circumference at baseline were less likely to reduce their modelled risk over the year. Smoking status and body mass index (BMI) were not associated with change in modelled risk. Stratifying by intervention group showed largely similar results, with a few exceptions. High baseline BMI in the RC group was associated with a smaller change in modelled risk. The association between large baseline waist circumference and CVD risk was only present in the RC group. Male sex was associated with smaller relative reductions in modelled risk, particularly in the RC group (Table 2).
Table 2.
Baseline predictors of change in modelled 10-year cardiovascular risk score (UKPDS risk engine version 3) in the ADDITION-Cambridge cohort
| Variable | n (%) | Beta coefficienta | (95% CI) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 2.36 (40) | — | — | — |
| Male | 3.61 (60) | 3.84 | 2.28 to 5.39 | <0.01 |
| Age at diagnosis, continuous variable | 0.18 | 0.09 to 0.27 | <0.01 | |
| Education, age finished, years | ||||
| <16 | 2.71 (45) | — | — | — |
| 16–18 | 246 (41) | –0.61 | –1.92 to 0.71 | 0.36 |
| >18 | 71 (12) | –0.62 | –2.64 to 1.40 | 0.54 |
| Smoking | ||||
| Never | 2.38 (40) | — | — | — |
| Former | 260 (44) | 1.29 | –0.20 to 2.78 | 0.09 |
| Current | 99 (17) | 0.95 | –0.39 to 2.28 | 0.16 |
| BMI, baseline | 0.02 | –0.08 to 0.12 | 0.68 | |
| Waist circumference, continuous variable | 0.05 | 0.01 to 0.09 | 0.01 | |
| Ethnicity | ||||
| White | 578 (97) | — | — | — |
| Asian | 17 (3) | –3.20 | –5.01 to –1.40 | <0.001 |
| Social class | ||||
| Professional | 31 (5) | — | — | — |
| Managerial and technical | 204 (34) | –0.32 | –3.09 to 2.45 | 0.82 |
| Skilled occupation: non-manual | 93 (16) | –2.94 | –5.93 to 0.05 | 0.05 |
| Skilled occupation: manual | 152 (25) | –0.01 | –3.15 to 3.14 | 1.00 |
| Partly skilled | 68 (11) | –0.91 | –3.55 to 1.72 | 0.49 |
| Unskilled | 38 (6) | –1.18 | –4.67 to 2.32 | 0.50 |
| Civil status | ||||
| Unmarried | 460 (77) | — | — | — |
| Married | 36 (6) | –2.01 | –4.74 to 0.72 | 0.15 |
| Divorced/separated | 54 (9) | –0.77 | –2.62 to 1.09 | 0.41 |
| Widow/widower | 45 (8) | –0.94 | –1.66 to 3.54 | 0.47 |
BMI = body mass index. CVD = cardiovascular disease. CVD risk change = follow-up CVD risk — baseline CVD risk.
Beta-coefficients denote the association between baseline predictors and CVD risk change adjusted for baseline CVD risk, treatment group, and clustering by practice. Numbers do not add up to total due to missing data.
Characteristics of treatment associated with change in modelled CVD risk
Prescription of higher numbers of drugs over the follow-up period was associated with larger reductions in modelled risk (Figure 2). In particular, risk reduction was greater among patients initiated on statin and antidiabetic medication. This association was not seen for the initiation of antihypertensive therapy, nor for an increase in the number of antihypertensive drugs prescribed. Individuals who decreased their energy intake, reduced their weight, and stopped smoking demonstrated more modelled CVD risk reduction than those who did not (Table 3). There was no association between change in self-reported physical activity, change in fruit and vegetable consumption (reported as change in plasma vitamin C concentration32), number of healthcare consultations during the previous 3 months, or self-reported medication adherence, and change in modelled risk. Results were unchanged by stratifying for treatment group and sex and when those with a prior CVD event were included.
Figure 2.
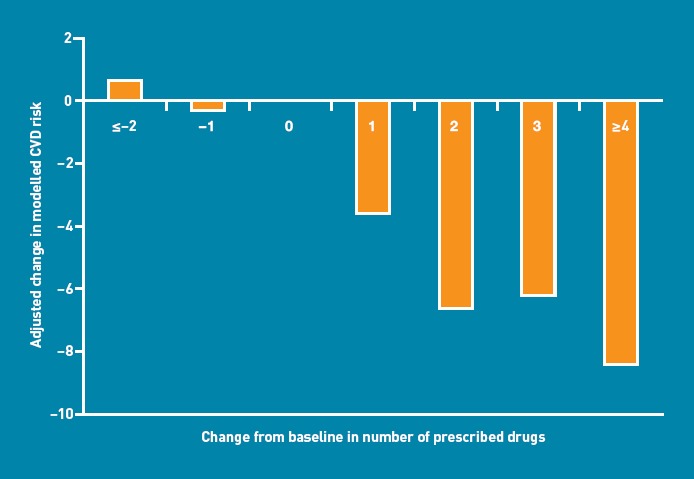
Change in modelled 10-year cardiovascular disease (CVD) risk (UKPDS risk engine version 3). CVD risk change = follow-up CVD risk — baseline CVD risk, adjusted for baseline CVD risk, treatment group, and taking cluster effect into account, in relation to change in total numbers of prescribed cardiovascular drugs from baseline to 14 months’ follow-up.
Table 3.
Association between lifestyle, intervention components, and change in modelled 10-year cardiovascular disease risk (UKPDS risk engine version 3) in the ADDITION-Cambridge cohort
| Variable | n (%) | Beta coefficienta | (95% CI) | P-value |
|---|---|---|---|---|
| Physical activity change, hours per week | ||||
| Total | –0.01 | –0.02 to 0.01 | 0.24 | |
| Recreational | –0.01 | –0.05 to 0.02 | 0.33 | |
| Vigorous | 0.08 | –0.17 to 0.33 | 0.51 | |
| Change in serum vitamin C | –0.01 | –0.04 to 0.02 | 0.50 | |
| Smoking | ||||
| No change (never, former) | 493 (80) | — | — | — |
| No change (smoking) | 86 (14) | 0.76 | –0.45 to 1.97 | 0.21 |
| Started | 5 (1) | 11.77 | 7.55 to 15.99 | <0.001 |
| Stopped | 13 (2) | –3.38 | –6.25 to –0.51 | 0.02 |
| Weight change, continuous variable (kg) | 0.25 | 0.13 to 0.37 | <0.001 | |
| Energy intake, continuous variable (kcal/day) | 0.12 | 0.03 to 0.22 | 0.01 | |
| Number of consultations in the last 3 months | ||||
| 0 | 71 (12) | — | — | — |
| 1 | 68 (11) | –2.2 | –4.95 to 0.54 | 0.12 |
| 2 | 114 (9) | –1.73 | –4.41 to 0.95 | 0.20 |
| 3 | 75 (13) | –1.28 | –4.35 to 1.79 | 0.42 |
| ≥4 | 96 (16) | –1.84 | –4.73 to 1.06 | 0.23 |
| Medical adherence at 1 year | ||||
| General (MARS ≤23) | 96 (16) | — | — | — |
| General (MARS >24) | 453 (76) | –0.89 | –2.55 to 0.76 | 0.28 |
| Change in medication | ||||
| Total drug number change | ||||
| ≤–2 | 13 (2) | 0.66 | –6.43 to 7.74 | 0.85 |
| –1 | 44 (7) | –0.30 | –2.71 to 2.12 | 0.81 |
| 0 | 181 (30) | — | — | — |
| 1 | 132 (22) | –3.59 | –5.31 to –1.86 | <0.001 |
| 2 | 127 (21) | –6.64 | –8.38 to –4.89 | <0.001 |
| 3 | 62 (10) | –6.20 | –8.54 to –3.86 | <0.001 |
| ≥4 | 38 (6) | –8.43 | –11.48 to –5.38 | <0.001 |
| Blood pressure drug change | ||||
| Never | 197 (33) | — | — | — |
| Prescribed | 94 (16) | –1.23 | –3.33 to 0.88 | 0.25 |
| Increased number | 87 (15) | –0.29 | –3.18 to 2.60 | 0.84 |
| Lipid-lowering change | ||||
| Never | 259 (43) | — | — | — |
| Prescribed | 242 (41) | –5.99 | –7.21 to –4.77 | <0.001 |
| Antidiabetic drugs | ||||
| Never | 417 (70) | — | — | — |
| Prescribed | 180 (30) | –3.94 | –5.66 to –2.22 | <0.001 |
CVD = cardiovascular disease. CVD risk change = follow-up CVD risk – baseline CVD risk.
Beta-coefficients denote the association between baseline predictors and CVD risk change adjusted for baseline CVD risk, treatment group, and clustering by practice. Numbers do not add up to total due to missing data.
Several patients were not prescribed medication despite elevated CVD risk factors. One hundred and twenty-seven (21%) patients had HbA1c 7% or more at follow-up but only 63 (50%) of these were prescribed medication to lower blood glucose. One hundred and thirty-two people (22%) had a total cholesterol above 5 mmol/l at follow-up but were not prescribed lipid-lowering therapy, and 67 participants (11%) were not prescribed antihypertensive treatment even though they had systolic blood pressure above 140 mmHg or diastolic blood pressure above 90 mmHg at follow-up. Among those with elevated blood pressure and HbA1c, males were less likely to be prescribed treatment than females, with no sex difference for lipid-lowering treatment. Overall, female patients were prescribed the same number of drugs as males and reported the same change in number of drugs as males. Females had a larger decrease in weight compared to males (–4.2 kg versus –3.3 kg); 32% of females reduced their weight by more than 6 kg compared to 24% of males.
DISCUSSION
Summary
A downward shift was observed in the distribution of modelled 10-year CVD risk in the 14 months following diagnosis, in a population of patients with screen-detected type 2 diabetes in the east of England. Modelled CVD risk declined by 5.3% on average; this reduction was not restricted to participants with the highest baseline CVD risk. This is an important finding, as the overall population effect of treatment in the lead time between detection by screening and clinical diagnosis is a key determinant of the cost effectiveness of a screening programme.33 Furthermore, the number of people with screen-detected diabetes is likely to rise following introduction of risk assessment in the NHS Health Checks programme.34 CVD risk reduction was mainly driven by prescription of a higher number of drugs, weight loss, and decreased energy intake over the follow-up period. Results of the study showed some variation in treatment and CVD risk reduction by age, sex, ethnicity, and obesity status. Taken together, the results support the suggestion that earlier detection and primary care treatment reduce cardiovascular risk.
Strength and limitations
Data on both practitioner and patient behaviour were collected from ADDITION-Cambridge, which allowed examination of the association between patient characteristics, treatment components, and CVD risk reduction. Nearly half of all practices approached agreed to participate.20 General practice registers typically cover 99% of all residents living in England.35 As such, ADDITION-Cambridge participants were drawn from a large population-based sample, ensuring generalisability to similar settings. However, extrapolation of the results to more-deprived settings with greater ethnic diversity may be limited in light of the non-random recruitment of general practices and trial participants from a single geographical region (eastern England). While deprivation scores were broadly similar between participating practices, ADDITION practices serve fewer deprived communities than the average English practice.21
The study data are observational and should thus be interpreted with caution. The outcome measure was change in 10-year modelled CVD risk. While the UKPDS risk engine exhibits reasonable predictive performance,36 it is not possible to be certain that the modelled CVD risk reduction seen in this cohort will translate into a reduction in hard CVD endpoints. Furthermore, the UKPDS score includes HbA1c, cholesterol, and blood pressure; medications that reduce these CVD risk factors are also likely to reduce modelled CVD risk. Measures of change in patient behaviour were calculated from self-reports of medication adherence, physical activity, and food intake, which may be subject to more error and bias than anthropometric and biochemical measures.
Comparison with existing literature
Several studies have shown that it is possible to achieve reduction in modelled CVD risk across the whole distribution of risk after clinical diagnosis of type 2 diabetes.3,6,37,38 The present study extends this finding to screen-detected patients. Results from the Dutch arm of the ADDITION-Europe trial support these findings, as significant reductions in several CVD risk factors were observed in both treatment groups after 1 year of follow-up.8
Prescribing increasing numbers of drugs to individuals with clinical diabetes has a significant impact on CVD risk reduction,3,38,39 a finding that may now be extended to modelled CVD risk among people with screen-detected diabetes. While prescription of lipid-lowering or antidiabetic drugs was associated with CVD risk reduction in the present study cohort, prescribing or increasing antihypertensive medication was not. The risk algorithm used to select high-risk individuals for screening included antihypertensive medication as a variable; therefore, 51% of the cohort were already prescribed antihypertensive medication at baseline. Further, patients prescribed these drugs during follow-up had a larger change in unadjusted risk score than individuals who were not prescribed these drugs. This was due to high CVD risk at baseline, suggesting that the main impact of antihypertensive medication was in the higher-risk group. Increased prescribing was not associated with an increased risk of mortality or hypoglycaemic episodes, suggesting that early treatment of individuals with screen-detected diabetes does not cause harm.40
Intensive lifestyle interventions, including promotion of physical activity, have been associated with clinically significant reductions in weight and improvements in several CVD risk factors.41,42 It was found in the present study that weight reduction and reduced daily energy intake, but not physical activity, were related to reduction in modelled CVD risk. Two recent studies show that weight reduction and diet may be more important than physical activity in terms of reducing hypertension and improving glycaemic control.35,43 However, the lack of effect of physical activity might also be explained by differential measurement error and bias; for example, changes in patient behaviour appeared to be quite small and were probably measured less precisely than, for example, prescribed medication.
Male patients were less likely to reduce their modelled CVD risk than females. No difference were found in levels of prescribed medication between males and females, but males were over-represented in groups with untreated elevated risk factors, indicating that additional risk reduction may have been possible in this group. These results are contrary to previous findings, where females with diabetes are reported to receive less medication and have poorer control of important modifiable risk factors than males.17,18,44 It was also found that reductions in modelled CVD risk were less likely among older participants, despite their having a higher level of CVD risk factors. This supports previous literature, in which patients aged >65 years with CVD had a reduced chance of being prescribed statins than younger individuals in England,15 suggesting that GPs may be reluctant to intensively treat older patients. The 17 Asian participants included in the present study demonstrated a larger risk reduction than their white counterparts, indicating that practitioners might be more vigilant in the treatment of this ethnic group. However, results should be interpreted with caution given the small numbers. In the RC group, individuals with a larger waist circumference and larger BMI at baseline were less likely to reduce their CVD risk. Clinicians admit to having negative attitudes towards obese patients,14 and obese patients report discrimination and poorer treatment from health providers.45,46 However, this finding was not observed in the IT group, suggesting that the education of primary care teams and the use of target-driven treatment algorithms may have reduced the potential for inequity of provision of treatment.
Implications for practice
It is possible to shift the distribution of modelled CVD risk in the first year after diagnosis in a population with screen-detected type 2 diabetes treated in primary care. This risk reduction is likely to be of clinical significance and, in this study, was mainly driven by prescription of a higher number of drugs, weight loss, and decreased energy intake. There is some suggestion that males with higher CVD risk were less intensively treated than females, especially in the control group. Even in this selected trial cohort there was room for further risk reduction as many patients were not prescribed recommended treatments.
Acknowledgments
We gratefully acknowledge the contribution of all participants, practice nurses and GPs in the ADDITION-Cambridge study. We also acknowledge the contribution of the trial steering committee (Professors Nigel Stott [Chair], John Weinman, Richard Himsworth, and Paul Little). The General Practice and Primary Care Research Unit at the University of Cambridge and the Medical Research Council Epidemiology Unit in Cambridge jointly coordinated the study. Aside from the authors, the ADDITION study team has included Amanda Adler, Ros Barling, Ryan Butler, Sean Dinneen, Mark Evans, Tom Fanshawe, Francis Finucane, Julie Grant, Wendy Hardeman, Robert Henderson, Richard Parker, Nicola Popplewell, Lincoln Sargeant, Stephen Sutton, and Fiona Whittle. We thank the Cambridge University Hospitals NHS Foundation Trust Department of Clinical Biochemistry and the NIHR Cambridge Biomedical Research Centre, Core Biochemical Assay Laboratory for carrying out the biochemical assays and the following groups within the MRC Epidemiology Unit: data management (Adam Dickinson), IT (Iain Morrison), technical (Matt Simms) and field epidemiology (Paul Roberts). Permission to use the ADDQol, DTSQ and well-being questionnaires was granted by Professor Clare Bradley, Health Psychology Research, Royal Holloway University of London, Surrey, UK.
Funding
ADDITION-Cambridge is supported by the Wellcome Trust (reference: G061895), the Medical Research Council (reference: G0001164), NHS R&D support funding, and the National Institute for Health Research. Ann-Louise Kinmonth, Kate M Williams, and Simon J Griffin (SJG) were members of the National Institute for Health Research (NIHR) School for Primary Care Research. The General Practice and Primary Care Research Unit is supported by NIHR Research funds. SJG receives support from the Department of Health NIHR Programme Grant funding scheme (RP-PG-0606–1259). The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Ethical approval
Ethical approval was granted by the Eastern Multi-Regional Ethics Committee (02/5/54).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
REFERENCES
- 1.Borch-Johnsen K, Lauritzen T, Glumer C, Sandbaek A. Screening for type 2 diabetes — should it be now? Diabet Med. 2003;20(3):175–181. doi: 10.1046/j.1464-5491.2003.00842.x. [DOI] [PubMed] [Google Scholar]
- 2.Wareham NJ, Griffin SJ. Should we screen for type 2 diabetes? Evaluation against National Screening Committee criteria. BMJ. 2001;322(7292):986–988. doi: 10.1136/bmj.322.7292.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 4.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 5.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355(9200):253–259. [PubMed] [Google Scholar]
- 6.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauritzen T, Griffin S, Borch-Johnsen K, et al. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000;24(suppl):S6–11. doi: 10.1038/sj.ijo.0801420. [DOI] [PubMed] [Google Scholar]
- 8.Janssen PG, Gorter KJ, Stolk RP, Rutten GE. Randomised controlled trial of intensive multifactorial treatment for cardiovascular risk in patients with screen-detected type 2 diabetes: 1-year data from the ADDITION Netherlands study. Br J Gen Pract. 2009;59(558):43–48. doi: 10.3399/bjgp09X394851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juul L, Sandbaek A, Foldspang A, et al. Adherence to guidelines in people with screen-detected type 2 diabetes, ADDITION, Denmark. Scand J Prim Health Care. 2009;27(4):223–231. doi: 10.3109/02813430903279117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmot MG, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 11.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: US trends from 1999 to 2006 and effects of medicare coverage. Ann Intern Med. 2009;150(8):505–515. doi: 10.7326/0003-4819-150-8-200904210-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7(1):29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 13.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 14.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17(5):941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 15.Reid FD, Cook DG, Whincup PH. Use of statins in the secondary prevention of coronary heart disease: is treatment equitable? Heart. 2002;88(1):15–19. doi: 10.1136/heart.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson CR, Hannaford PC, Williams D. Evidence for inequalities in the management of coronary heart disease in Scotland. Heart. 2005;91(5):630–634. doi: 10.1136/hrt.2004.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legato MJ, Gelzer A, Goland R, et al. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3(2):131–158. doi: 10.1016/s1550-8579(06)80202-0. [DOI] [PubMed] [Google Scholar]
- 18.Tabenkin H, Eaton CB, Roberts MB, et al. Differences in cardiovascular disease risk factor management in primary care by sex of physician and patient. Ann Fam Med. 2010;8(1):25–32. doi: 10.1370/afm.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340(8):618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 20.Echouffo-Tcheugui JB, Simmons RK, et al. The ADDITION-Cambridge trial protocol: a cluster — randomised controlled trial of screening for type 2 diabetes and intensive treatment for screen-detected patients. BMC Public Health. 2009;9:136. doi: 10.1186/1471-2458-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sargeant LA, Simmons RK, Barling RS, et al. Who attends a UK diabetes screening programme? Findings from the ADDITION-Cambridge study. Diabet Med. 2010;27(9):995–1003. doi: 10.1111/j.1464-5491.2010.03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh A, Hutchinson A, Home PD, et al. Clinical guidelines and evidence review for type 2 diabetes: management of blood glucose. Sheffield: ScHARR, University of Sheffield; 2001. [Google Scholar]
- 24.McIntosh A, Hutchinson A, Home PD, et al. Clinical guidelines and evidence for type 2 diabetes: management of blood pressure. Sheffield: ScHARR, University of Sheffield; 2002. [Google Scholar]
- 25.McIntosh A, Hutchinson A, Homne PD, et al. Clinical guidelines and evidence for type 2 diabetes: lipids management. Sheffield: ScHARR, University of Sheffield; 2010. [Google Scholar]
- 26.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 27.Pyorala K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) Diabetes Care. 1997;20(4):614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 28.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 29.Home P, Weinman J. Predicting treatment adherence: an overview of theoretical models. In: Myers L, Midence K, editors. Adherence to treatment in medical conditions. London: Harwood Academic; 1998. pp. 25–50. [Google Scholar]
- 30.Wareham NJ, Jakes RW, Rennie KL, et al. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol. 2002;31(1):168–174. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- 31.Bingham SA, Gill C, Welch A, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(Suppl 1):S137–S151. doi: 10.1093/ije/26.suppl_1.s137. [DOI] [PubMed] [Google Scholar]
- 32.Harding AH, Wareham NJ, Bingham SA, et al. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer — Norfolk prospective study. Arch Intern Med. 2008;168(14):1493–1499. doi: 10.1001/archinte.168.14.1493. [DOI] [PubMed] [Google Scholar]
- 33.Glumer C, Yuyun M, Griffin S, et al. What determines the cost-effectiveness of diabetes screening? Diabetologia. 2006;49(7):1536–1544. doi: 10.1007/s00125-006-0248-x. [DOI] [PubMed] [Google Scholar]
- 34.Department of Health. Putting prevention first — vascular checks: risk assessment and management. next steps guidance for primary care trusts. London: Department of Health; 2009. [Google Scholar]
- 35.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378(9786):129–139. doi: 10.1016/S0140-6736(11)60442-X. [DOI] [PubMed] [Google Scholar]
- 36.Simmons RK, Coleman RL, Price HC, et al. Performance of the UK Prospective Diabetes Study Risk Engine and the Framingham Risk Equations in Estimating Cardiovascular Disease in the EPIC-Norfolk Cohort. Diabetes Care. 2009;32(4):708–713. doi: 10.2337/dc08-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivarius NF, Beck-Nielsen H, Andreasen AH, et al. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ. 2001;323(7319):970–975. doi: 10.1136/bmj.323.7319.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calles-Escandon J, Lovato LC, Simons-Morton DG, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the ACCORD trial. Diabetes Care. 2010;33(4):721–727. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 40.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigal RJ, Kenny GP, Wasserman DH, et al. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Das S, Barlow CE, et al. Fitness, fatness, and systolic blood pressure: data from the Cooper Center Longitudinal Study. Am Heart J. 2010;160(1):166–170. doi: 10.1016/j.ahj.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Gouni-Berthold I, Berthold HK, Mantzoros CS, et al. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2008;31(7):1389–1391. doi: 10.2337/dc08-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaminsky J, Gadaleta D. A study of discrimination within the medical community as viewed by obese patients. Obes Surg. 2002;12(1):14–18. doi: 10.1381/096089202321144513. [DOI] [PubMed] [Google Scholar]
- 46.Fontaine KR, Faith MS, Allison DB, Cheskin LJ. Body weight and health care among women in the general population. Arch Fam Med. 1998;7(4):381–384. doi: 10.1001/archfami.7.4.381. [DOI] [PubMed] [Google Scholar]


