Abstract
Hematopoietic stem cell transplant patients are susceptible to infection despite cellular reconstitution. In a murine model of syngeneic bone marrow transplantation (BMT), we previously reported that BMT mice have impaired host defense against Pseudomonas aeruginosa pneumonia due to overproduction of (PG)E2 in lung. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is an effector in the PGE2 signaling pathway that negatively regulates alveolar macrophage (AM) phagocytosis and bacterial killing. Therefore, examined whether overproduction of PGE2 after BMT inhibits AM host defense by up-regulating PTEN phosphatase activity. We found that PTEN activity is elevated in BMT AMs in response to increased PGE2 signaling and that pharmacological inhibition of PTEN activity in BMT AMs fully restores phagocytosis of serum-opsonized P. aeruginosa but only partially restores phagocytosis of nonopsonized P. aeruginosa. In wild-type mice transplanted with myeloid-specific conditional PTEN knockout (PTEN CKO) bone marrow, bacterial clearance is improved after challenge with P. aeruginosa pneumonia. Furthermore, PTEN CKO BMT AMs display improved TNF-α production and enhanced phagocytosis and killing of serum-opsonized P. aeruginosa despite overproduction of PGE2. However, AM phagocytosis of nonopsonized P. aeruginosa is only partially restored in the absence of PTEN after BMT. This may be related to elevated AM expression of IL-1 receptor–associated kinase (IRAK)-M, a molecule previously identified in the PGE2 signaling pathway to inhibit AM phagocytosis of nonopsonized bacteria. These data suggest that PGE2 signaling up-regulates IRAK-M independently of PTEN and that these molecules differentially inhibit opsonized and nonopsonized phagocytosis of P. aeruginosa.
Keywords: pneumonia, lung, neutrophil, eicosanoids, bacteria
Clinical Relevance
Our results demonstrate that transplant-induced prostaglandin E2 may differentially inhibit opsonized and nonopsonized phagocytosis in alveolar macrophages via the up-regulation of phosphatase and tensin homolog deleted on chromosome 1 activity and IL-1 receptor–associated kinase M expression, respectively. Our studies provide a rationale for prostaglandin blockade as a therapy for pulmonary bacterial infections after stem cell transplant.
Hematopoietic stem cell transplantation (HSCT) is commonly used for the treatment of various malignant and genetic disorders of the immune system. However, delays in immune reconstitution and reduced functionality of recovered immune cell compartments render patients susceptible to infectious complications, including bacterial pneumonia (1–3). This impairment in immune recovery and function extends for several months after transplant and occurs in autologous and allogeneic transplant recipients (4, 5).
Alveolar macrophages (AMs) are the primary immune cell type in the alveolar space and are critical for initiating immune responses against inhaled pathogens (6–8). However, AMs are reported to have decreased host defense capability after allogeneic HSCT (9). Furthermore, neutrophils recruited to sites of infection display impaired chemotaxis and killing ability (10). These studies using allogeneic transplant patients, however, involve various confounding factors, such as immunosuppressive drug therapy and graft-versus-host disease, that are known to impair immune function (3, 4). Thus, the specific impact of conditioning and reconstitution alone on pulmonary innate immune function remains unclear.
To determine the impact of HSCT on pulmonary host defense, we previously developed a mouse model of syngeneic bone marrow transplantation (BMT). Compared with nontransplant control mice, BMT mice were more susceptible to P. aeruginosa pneumonia after intratracheal infection despite full hematopoietic reconstitution in the lung and periphery (11). Furthermore, donor-derived BMT AMs and recruited lung neutrophils displayed impaired host defense functions (12). We found that this reduction in innate immune function was induced by an elevated production of the immunosuppressive lipid mediator prostaglandin (PG)E2 in the lung after BMT (2, 12, 13).
PGE2 is known to inhibit bacterial killing, phagocytosis (14, 15), chemotaxis (16), and the production of proinflammatory mediators in leukocytes (17–19). At least one consequence of increased PGE2 production after transplant is the up-regulation of IL-1 receptor–associated kinase (IRAK)-M, which limits AM function (including inhibition of phagocytosis of nonopsonized P. aeruginosa) (20). However, it is not known what downstream signaling pathways PGE2 activates to up-regulate IRAK-M expression. One potential candidate in the PGE2 signaling pathway that may regulate IRAK-M is the phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which has also been reported to negatively regulate Fcγ receptor (FcγR)-mediated phagocytosis and bacterial killing in AMs (21). It remains unknown whether PTEN regulates AM function in the BMT setting.
PTEN is a dual-specificity phosphatase that can dephosphorylate protein and lipid targets; however, PTEN is best characterized by its ability to dephosphorylate the lipid second messenger phosphatidylinositol (3–5)-trisphosphate (PIP3), a key mediator of the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway (22). This dephosphorylation event results in the inhibition of FcγR-mediated phagocytosis of IgG-opsonized particles as mediated by PI3K/AKT signaling (23, 24). PTEN phosphatase activity is negatively regulated via a number of posttranslational mechanisms, including tyrosine phosphorylation (25). PGE2 activation of the cAMP signaling cascade has been shown to induce PTEN activity via tyrosine dephosphorylation in a Src homology region 2 domain–containing phosphatase–1 dependent manner (21, 26). Furthermore, these studies showed that PGE2–mediated increases in PTEN activity diminish AM phagocytosis and killing of IgG-opsonized particles (21). Additional roles for PTEN may exist in the inhibition of non–FcγR-mediated phagocytosis, given that PI3K activity has been reported to mediate phagocytosis of nonopsonized particles (27–29). Therefore, we hypothesized that, in our model of syngeneic BMT, increased PGE2 signaling in the lung inhibits host defense against acute P. aeruginosa infection by elevating PTEN activity in AMs. Additionally, we wished to determine the influence of PTEN in opsonized and nonopsonized phagocytosis pathways and whether PTEN signaling is related to IRAK-M elevation after BMT.
To address our hypothesis, we measured PTEN activity and AKT phosphorylation levels in BMT and in nontransplant control AMs in the presence or absence of an inhibitor of endogenous PGE2 production. In addition, we transplanted lethally irradiated wild-type (WT) mice with bone marrow from myeloid-specific PTEN conditional knockout (CKO) mice to determine whether PTEN plays a role in impaired pulmonary host defense after BMT. We demonstrate that increased PGE2 signaling augments PTEN activity in BMT AMs and diminishes pAKT levels. Furthermore, we show that myeloid-specific ablation of PTEN in the bone marrow of transplant mice can restore AM phagocytosis of serum-opsonized bacteria and improve bacterial clearance after P. aeruginosa infection. In contrast, PTEN CKO BMT AMs do not have fully restored nonopsonized phagocytosis of P.aeruginosa, likely due to the up-regulation of IRAK-M in these phagocytes. Taken together, our results demonstrate that, after BMT, PGE2 signals through IRAK-M independently of PTEN and that up-regulation of PTEN activity primarily inhibits serum-opsonized phagocytosis, whereas up-regulation of IRAK-M may specifically inhibit nonopsonized phagocytosis. Additionally, results from our PTEN CKO BMT studies demonstrate the importance of AM function for in vivo clearance of P. aeruginosa independent of neutrophil function.
Materials and Methods
Additional details regarding all methods can be found in the online supplement.
Animals
WT C57BL/6 (B6), PTENloxP/loxP, and myeloid-specific LysM Cre mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Myeloid-specific PTEN KO mice (PTENloxP/loxP;Cre+/−) were generated by breeding as previously described (30). For all experiments involving myeloid-specific PTEN KO mice, PTENwt/wt;Cre+/− mice were used as WT bone marrow donors and PTENloxP/loxP;Cre+/− mice were used as PTEN CKO bone marrow donors. Mice were housed under specific pathogen–free conditions and monitored daily by veterinary staff. All mice were killed by CO2 asphyxiation. The University of Michigan Committee on Use and Care of Animals approved these experiments.
BMT
Total body irradiation and BMT were performed as previously described (20). All experiments with BMT mice were performed 5 to 6 weeks after BMT when mice were fully donor-cell reconstituted (13, 31).
P. aeruginosa PAO1 Preparation and Intratracheal Infection
As previously described, P. aeruginosa PAO1 inoculum was prepared, and mice were injected intratracheally with a sublethal dose of 5 × 105 CFU (12, 31).
Immune Serum Preparation and Opsonization
P. aeruginosa–specific immune serum was prepared (without heat inactivation) and used to opsonize P. aeruginosa as previously described (32).
Quantification of Bacterial Burden in Lung and Blood
Bacterial burden in whole lung and blood samples was assessed by CFU assay as previously described (12).
AM and Neutrophil Isolation
AMs and elicited lung neutrophils were harvested by bronchoalveolar lavage (BAL), counted, and adherence purified as previously described (31).
IgG-Sheep Red Blood Cell FcγR Stimulation Assay
AMs were pretreated with the drugs of interest, stimulated at a 1:10 ratio with IgG-opsonized or nonopsonized sheep red blood cells (MP Biomedicals, Solon, OH), and prepared for Western blot analysis as described previously (21, 33).
Differential Cell Analysis of Total Lung Leukocytes and BAL Cells
Differential analysis was performed using BAL cells or total lung leukocytes isolated from collagenase-digested whole lung samples as previously described (20).
In Vitro Phagocytosis and Bacterial Killing Assays
AM phagocytosis of FITC-labeled P. aeruginosa was measured in vitro as previously described (20). Bacterial killing was quantified in AMs and lung neutrophils in vitro using a tetrazolium dye reduction assay as described previously (34).
Western Blot Analysis
Western blot analysis was performed as previously described (20, 21) using 15 μg of whole-cell lysate or protein isolated by immunoprecipitation.
PTEN Phosphatase Activity Assay
PTEN was immunoprecipitated from AM whole-cell lysates to assess in vitro lipid phosphastase activity as previously described (21, 35).
ELISA/Enzyme-Linked Immunoassay
Enzyme-linked immunoassay samples were prepared as previously described (20). TNF-α production was measured using a DuoSet ELISA kit (R&D Systems, Minneapolis, MN), and PGE2 production was measured using a PGE2 enzyme-linked immunoassay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
Statistical Analysis
Statistical significance was analyzed using the Prism 5.01 (GraphPad Software, San Diego, CA). ANOVA with a post hoc Bonferroni test was used to compare groups as previously described (20). A value of P < 0.05 was considered statistically significant.
Results
Increased PTEN Activity and Diminished pAKT Levels in BMT AMs
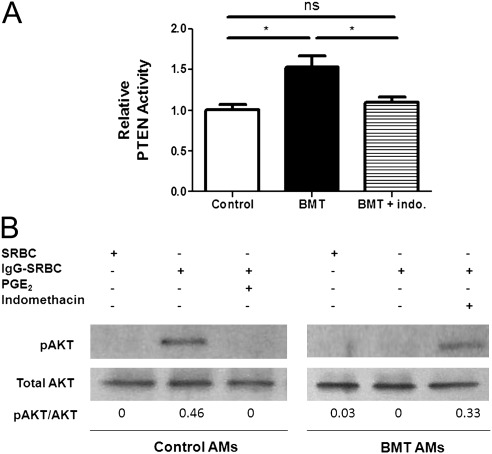
After syngeneic BMT, AMs display elevated production of PGE2, which directly impairs AM phagocytosis and killing of IgG-opsonized and nonopsonized particles (12). Given that PGE2 is known to inhibit FcγR-mediated phagocytosis and killing in AMs by up-regulating PTEN activity (21), we determined whether overproduction of PGE2 in BMT AMs results in elevated PTEN phosphatase activity. PTEN was immunoprecipitated from BMT and nontransplant control AMs, and the lipid phosphatase activity of the purified PTEN protein was assessed after incubation with PIP3. Relative to control AMs, BMT AMs had an approximately 50% increase in PTEN activity (Figure 1A). This increase was blocked in BMT AMs after overnight treatment with indomethacin, an inhibitor of endogenous prostanoid production (Figure 1A). Because PGE2 is the major prostanoid produced in BMT AMs (12, 13), the indomethacin treatment likely exerts its effect by blocking PGE2.
Figure 1.
Overproduction of prostaglandin (PG)E2 elevates phosphatase and tensin homolog deleted on chromosome 10 (PTEN) activity and diminishes pAKT levels in bone marrow transplantation (BMT) alveolar macrophages (AMs). Mice were given syngeneic BMT and harvested 5 to 6 weeks after transplant. AMs were isolated by bronchoalveolar lavage as described in Materials and Methods from BMT and nontransplanted control mice. (A) PTEN protein was immunoprecipitated from whole-cell lysates of AMs cultured at 5 × 105 per well in the presence of 5 μM indomethacin or 0.05% vehicle control overnight. PTEN phosphatase activity was determined as described in Materials and Methods. Results are represented as PTEN phosphatase activity relative to the untreated control group (*P < 0.05; n = 3–4 per group combined from two experiments). (B) AMs were pretreated with 5 μM indomethacin for 2 hours or 100 nM PGE2 for 15 minutes at 37°C. AMs were then stimulated with IgG–sheep red blood cells (SRBCs) (1:10 ratio) for 15 minutes at 37°C. After IgG-SRBC stimulation, AMs were prepared for Western blot analysis as described in Materials and Methods. Relative band densitometry data are indicated under each lane. Data shown are representative of two experiments.
PTEN activity is negatively regulated via a number of posttranslational mechanisms, including tyrosine phosphorylation (25). Our preliminary data suggest that the increase we observed in PTEN activity in BMT AMs is related, at least in part, to diminished PTEN tyrosine phosphorylation (see Fig. E1, lane 2, in the online supplement). Furthermore, these data suggest that the reduction in PTEN tyrosine phosphorylation is due to BMT AM overproduction of PGE2, given that PTEN tyrosine phosphorylation is improved in BMT AMs after treatment with indomethacin (Fig. E1, lane 3).
Increased PTEN activity inhibits the activation of a number of downstream targets in the FcγR-signaling pathway, such as AKT (22, 25). To determine whether increased PTEN activity in BMT AMs translates into functional Akt suppression, AMs from control and BMT mice were stimulated with IgG-opsonized sheep red blood cells to induce phosphorylation of AKT. FcγR stimulation of control AMs induced phosphorylation of AKT (Figure 1B, lane 2); however, as previously reported (21), pretreatment with 100 nM PGE2 blocked FcγR-induced AKT phosphorylation (Figure 1B, lane 3). As expected, BMT AMs had no detectable levels of pAKT after FcγR stimulation (Figure 1B, lane 5). pAKT levels were restored in FcγR-stimulated BMT AMs after pretreatment with indomethacin (Figure 1B, lane 6). Taken together, these data suggest that elevated PTEN activity and diminished pAKT levels in BMT AMs are related to increased PGE2 signaling.
Inhibition of PTEN Activity Restores Phagocytosis in BMT AMs
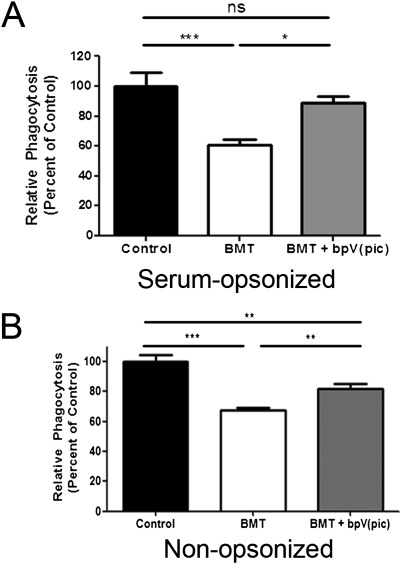
Because PTEN activity is known to inhibit FcγR-mediated phagocytosis in macrophages (21, 36), we determined whether pharmacologic inhibition of PTEN activity could restore phagocytosis in BMT AMs. The phosphatase inhibitor bpV(pic) specifically inhibits PTEN phosphatase activity at concentrations within the nanomolar range (37). We found that pretreating BMT AMs with 100 nM bpV(pic) restored phagocytosis of serum-opsonized P. aeruginosa to that of control AM levels (Figure 2A). In experiments using nonopsonized P. aeruginosa, bpV(pic) treatment was also able to improve the phagocytic function of BMT AMs, although not to control AM levels (Figure 2B). These data suggest that PTEN negatively regulates phagocytosis but may play a more important role in the inhibition of serum-opsonized phagocytosis as opposed to nonopsonized phagocytosis.
Figure 2.
Inhibiting PTEN activity improves BMT AM phagocytic ability. AMs were harvested from control and BMT mice and cultured at 2 × 105 cells per well overnight. Phagocytosis of serum-opsonized (A) and non–serum-opsosonized (B) FITC–P. aeruginosa was assessed as described in Materials and Methods (*P < 0.05, **P < 0.01, ***P < 0.001; panel A: n = 10 per group; panel B: n = 5 per group).
Myeloid-Specific Ablation of PTEN Expression in BMT Mice
Myeloid-specific ablation of PTEN enhances lung phagocyte function and pulmonary host defense against gram-negative bacterial infections (38); however, it is not known whether similar mechanisms are relevant in the setting of BMT. Because our data demonstrate that pharmacologic inhibition of PTEN activity improves BMT AM phagocytosis in vitro (Figure 2), we generated myeloid-specific PTEN KO mice to assess whether reconstituting irradiated WT mice with PTEN-deficient bone marrow would restore host defense function in AMs. To generate myeloid-specific PTEN KO mice, we obtained mice homozygous for a “floxed” PTEN mutant allele containing loxP sites on either side of exon 5 encoding the phosphatase domain. The PTEN floxed mice were bred to myeloid-specific Cre mice, which express the Cre recombinase gene under the control of the lysozyme M (LysM) promoter. As previously described, mice bred to express two copies of the floxed PTEN allele and at least one copy of LysM-Cre recombinase are deficient in PTEN expression in cells of the myeloid lineage (30). Furthermore, these mice do not display an abnormal phenotype and are viable, fertile, and of normal size (30).
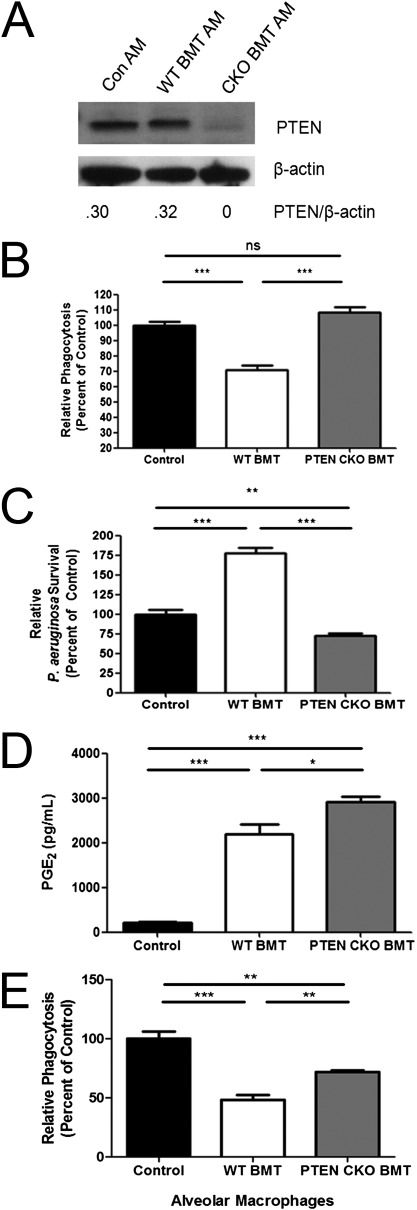
To verify PTEN ablation in our BMT model, AMs were harvested from nontransplanted WT control mice and lethally total body irradiated WT mice transplanted with WT BMT or myeloid-specific (conditional) PTEN CKO BMT. Figure E2 demonstrates that these cells isolated by BAL from each group of mice are predominantly AMs. PTEN protein expression was comparable in control and WT BMT AMs (Figure 3A). As expected, PTEN protein expression was ablated in AMs from PTEN CKO BMT mice (Figure 3A), and preliminary data suggest that this is accompanied by a restoration in basal pAKT expression levels (Fig. E3). Ablation of PTEN was also observed in recruited lung neutrophils from PTEN CKO BMT mice (data not shown).
Figure 3.
Restored AM phagocytosis and killing of serum-opsonized P. aeruginosa in PTEN conditional knockout (CKO) BMT mice despite AM overproduction of PGE2. (A) Whole-cell lysates were prepared from control, wild-type (WT) BMT, and PTEN CKO BMT AMs after 1 hour of serum-free media adherence. PTEN and β-actin protein expression were analyzed by Western blot. Ablation of PTEN was verified in this manner for all experiments using PTEN CKO BMT mice. (B and C) Bone marrow from WT or myeloid-specific PTEN CKO mice was transplanted into lethally irradiated WT recipients. WT control, WT BMT (WT > WT), and PTEN CKO BMT (PTEN CKO > WT) AMs were harvested and cultured at 2 × 105 cells per well overnight. Phagocytosis of serum-opsonized FITC–P. aeruginosa (B) and killing of serum-opsonized P. aeruginosa (C) was assessed as described in Materials and Methods (n = 7–10 per group). (D) PGE2 levels were measured in overnight culture supernatants by Enzyme-linked immunoassay as described in Materials and Methods (n = 4–5 per group). (E) AMs were harvested from control, WT BMT, and PTEN CKO BMT mice and cultured at 2 × 105 cells/well overnight. Phagocytosis of non–serum-opsosonized FITC P. aeruginosa was assessed (E) as described in Materials and Methods (n = 7–10 per group; *P < 0.05, **P < 0.01, ***P < 0.001).
AMs from PTEN CKO BMT Mice Display Enhanced Phagocytosis and Killing of Serum-Opsonized P. aeruginosa Despite Overproduction of PGE2
We performed experiments to determine whether myeloid-specific disruption of PTEN could improve opsonized phagocytosis and killing of serum-opsonized P. aeruginosa in BMT AMs. We found that AMs from PTEN CKO BMT mice displayed restored phagocytosis of serum-opsonized FITC–P. aeruginosa in vitro relative to WT BMT AMs (Figure 3B). Furthermore, WT BMT AMs had increased survival of ingested P. aeruginosa relative to control AMs, indicating defective bacterial killing ability (Figure 3C). However, the amount of surviving bacteria was significantly lower in PTEN CKO BMT AMs compared with control AMs, suggesting that bacterial killing is not only restored in AMs from PTEN CKO BMT mice but is also enhanced (Figure 3C). AMs from PTEN CKO BMT mice produce significantly more PGE2 than control and WT BMT AMs, as measured from overnight culture supernatants (Figure 3D). These data suggest that PTEN ablation in AMs restores phagocytosis of serum-opsonized bacteria after BMT despite AM overproduction of PGE2.
PTEN Ablation Improves but Does Not Fully Restore Nonopsonized Phagocytosis in AMs after BMT
Our results with the PTEN inhibitor bpV(pic) suggest that PTEN function may be less important for nonopsonized phagocytosis of bacteria. To verify this with the genetic ablation, AMs were harvested from control, WT BMT, and PTEN CKO BMT mice, and phagocytosis of nonopsonized bacteria was tested in vitro. Similar to our findings shown in Figure 2, PTEN CKO BMT AMs showed improved phagocytosis compared with WT BMT AMs (Figure 3E). However, this improvement in phagocytic ability was not restored to control AM levels (Figure 3E). Thus, BMT-mediated increases in PTEN may play a lesser role in nonopsonized phagocytosis as compared with opsonized phagocytosis.
Restored AM and Lung TNF-α Levels in PTEN CKO BMT Mice
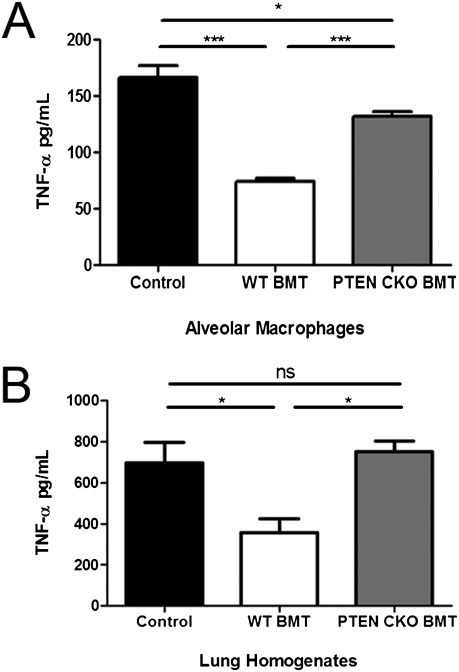
AMs from neutropenic myeloid-specific PTEN KO mice display enhanced proinflammatory cytokine profiles, including increased production of TNF-α, relative to neutropenic WT mice (38). Because TNF-α is a key proinflammatory mediator in pulmonary host defense against P. aeruginosa (8) and can enhance bacterial killing mechanisms in macrophages (39–41), we measured TNF-α levels in AM overnight culture supernatants and lung homogenates from mice challenged with an acute P. aeruginosa lung infection. As previously reported (11, 31), defective TNF-α production was observed in WT BMT AMs and lung homogenates relative to control samples (Figures 4A and 4B). However, PTEN CKO BMT mice displayed improved AM TNF-α production (Figure 4A), and lung homogenates from infected mice had fully restored TNF-α levels (Figure 4B).
Figure 4.
PTEN CKO BMT mice display improved TNF-α production. TNF-α production was measured by ELISA as described in Materials and Methods using AM overnight culture supernatants (A) and lung homogenates from mice 24 hours after intratracheal P. aeruginosa infection (B) (*P < 0.05, ***P < 0.001; n = 3–5 per group).
Improved Bacterial Clearance in PTEN CKO BMT Mice after P. aeruginosa Infection
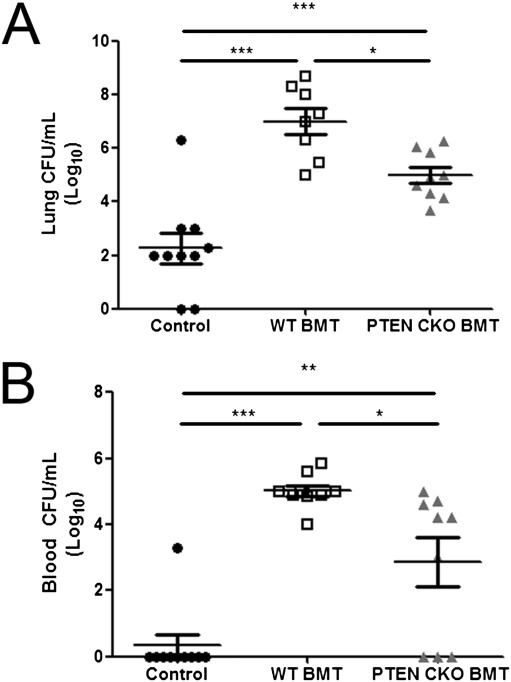
To determine whether PTEN CKO BMT mice have improved bacterial clearance after BMT, we challenged control, WT BMT, and PTEN CKO BMT mice with a sublethal P. aeruginosa lung infection. At 24 hours after infection, bacterial burden was assessed in lung and blood samples. In WT BMT mice, bacterial burden was significantly higher in lung and blood samples relative to control (Figures 5A and 5B). In PTEN CKO BMT mice, bacterial burden was significantly reduced in lung and blood samples relative to WT BMT mice, although it was not fully restored to control levels (Figures 5A and 5B). We speculate that the reason PTEN CKO BMT mice have an intermediate phenotype is that the acute in vivo infection model relies heavily on AM clearance of nonopsonized bacteria—a pathway in which PTEN may be less important.
Figure 5.
Improved bacterial clearance in PTEN CKO BMT mice after P. aeruginosa pneumonia. Mice were injected intratracheally with 50 μL of 5 × 105 CFU P. aeruginosa. At 24 hours after infection, bacterial burden of whole lung (A) and blood (B) samples from each mouse was assessed by CFU assay (*P < 0.05, **P < 0.01, ***P < 0.001; n = 8–10 mice per group).
IRAK-M Expression Is Elevated in WT BMT and PTEN CKO BMT AMs
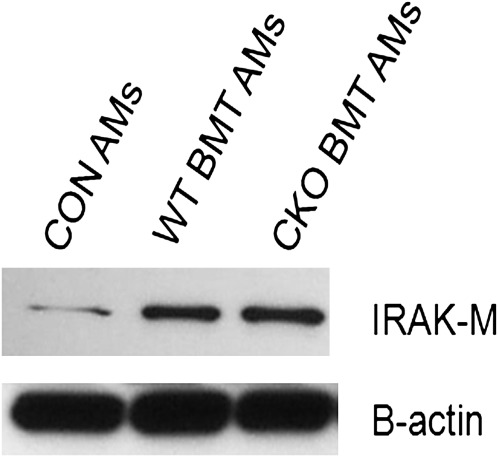
We previously demonstrated that, in response to increased PGE2 signaling, BMT AMs display elevated expression of IRAK-M, a well described negative regulator of TLR/IL-1R signaling (20). Furthermore, we found that, despite overproduction of PGE2, ablation of IRAK-M in the hematopoietic compartment of BMT mice fully restores BMT AM phagocytosis of nonopsonized bacteria, killing, and production of proinflammatory mediators (20). Therefore, we wondered whether the partial restoration of nonopsonized phagocytosis in PTEN CKO BMT AMs was the result of elevated IRAK-M expression. IRAK-M protein expression was assessed in AMs from control, WT BMT, and PTEN CKO BMT mice. IRAK-M protein expression was significantly elevated in WT BMT and PTEN CKO BMT AMs compared with control AMs (Figure 6). These data suggest that, after BMT, IRAK-M may play a critical role in the negative regulation of nonopsonized phagocytosis, whereas PTEN primarily inhibits pathways of opsonized phagocytosis.
Figure 6.
Elevated IL-1 receptor-associated kinase (IRAK)-M expression in WT BMT and PTEN CKO BMT AMs. Whole-cell lysates were prepared from pooled control, WT BMT, and PTEN CKO BMT AMs after 1 hour of serum-free media adherence. IRAK-M and β-actin protein expression were analyzed by Western blot as described in Materials and Methods. Blot is representative of data from two experiments.
Lung Leukocyte Composition in PTEN CKO BMT Mice
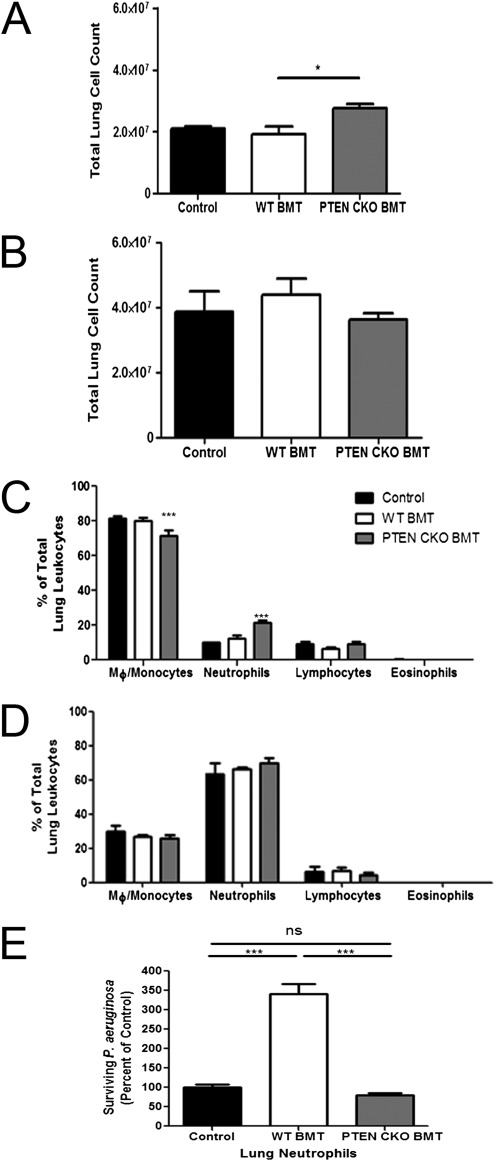
We next assessed the total number of leukocytes and the frequency of leukocyte populations in the lungs of control, WT BMT, and PTEN CKO BMT mice at baseline and 24 hours after P. aeruginosa pneumonia. Total lung leukocyte numbers were increased in uninfected PTEN CKO BMT mice relative to uninfected WT BMT mice (Figure 7A). However, 24 hours after infection, total leukocyte numbers were comparable between groups (Figure 7B). A shift was observed in whole lung macrophage and neutrophil counts in uninfected PTEN CKO BMT mice (Figure 7C). We found that the percentage of macrophages was significantly reduced and neutrophils increased relative to control and WT BMT lungs (Figure 7C). After infection, however, the percentages of neutrophils, macrophages, and lymphocytes were comparable between groups (Figure 7D).
Figure 7.
Lung leukocyte composition before and after P. aeruginosa infection and neutrophil function. Control, WT BMT, and PTEN CKO BMT mice were injected intratracheally with 50μL of 5 × 105 CFU P. aeruginosa. Lung samples were harvested from uninfected and infected mice 24 hours later, and lung leukocytes were isolated, counted, and stained for differential cell analysis as described in Materials and Methods. (A and B) Total lung leukocyte count in naive (A) and infected (B) mice. (C and D) Percentage of macrophages/monocytes, neutrophils, lymphocytes, and eosinophils in the lungs of naive (C) and infected (D) mice (n = 4 per group). Neutrophils were elicited to the lung and harvested by BAL from control, WT BMT, and PTEN CKO BMT mice as described in Materials and Methods. Killing of serum-opsonized P. aeruginosa was assessed in neutrophils (E) as described in Materials and Methods (n = 7–10 per group; * P < 0.05, *** P < 0.001).
Neutrophil Function Is Improved in PTEN CKO BMT Mice
Previous studies have suggested that PTEN-deficient neutrophils have improved host defense functions (38, 42). Given that PTEN CKO BMT mice have increased neutrophil numbers in the lung at baseline, we wanted to assess the functional status of these phagocytes with regard to bacterial killing. Relative to control neutrophils, WT BMT neutrophils displayed impaired bacterial killing of serum-opsonized P. aeruginosa, and this defect was restored in the absence of PTEN after BMT (Figure 7E). Thus, despite the fact that PTEN CKO BMT mice have increased numbers of neutrophils in the lung at baseline and display normal killing of serum-opsonized P. aeruginosa, the in vivo clearance of bacteria in these mice is still not completely restored. In total, these data suggest that persistent impairment of nonopsonized phagocytosis by AMs likely contributes to the partial restoration of host defense against acute P. aeruginosa lung infection in PTEN CKO BMT mice, despite an increased presence of functional neutrophils.
Discussion
AMs are the primary immune cell type in the alveolar space and serve as the first line of cellular defense against inhaled pathogens (6–8). Defects in AM phagocytosis/killing and production of inflammatory signals hinder the ability of the host to clear pathogens. In the case of HSCT, donor-derived AMs reconstituting the lung airspaces have a significantly impaired ability to ingest and kill bacterial pathogens (9, 11), leaving the host susceptible to organisms such as P. aeruginosa.
Our previous work has shown that overproduction of PGE2 BMT directly impairs AM host defense (12). One effector in the PGE2 signaling cascade, PTEN, has recently been shown to inhibit AM phagocytosis and killing of IgG-opsonized particles by negatively regulating the FcγR signaling pathway in control mice (21). In this work, we show a similar mechanism after BMT, where PGE2 is overproduced by AMs and increases the lipid phosphatase activity of PTEN (see Figure 1A). PTEN activity inhibits BMT AM phagocytosis of serum- and non–serum-opsonized P. aeruginosa (see Figure 2), although it appears that PTEN may play a more critical role in the negative regulation of opsonized phagocytosis. In addition, we show that myeloid-specific ablation of PTEN restores bacterial host defense functions in AMs (see Figure 3) and neutrophils (Figure 7E) after BMT.
Our data demonstrate that BMT AMs have increased PTEN activity relative to nontransplant control AMs (see Figure 1A). Because PGE2 has been shown to increase PTEN activity via activation of the cAMP signaling cascade, we examined whether BMT AM overproduction of PGE2 mediated this increase in PTEN activity. Indomethacin treatment, which inhibits COX-2 synthesis of prostaglandins, effectively abrogated the increase we observed in PTEN activity (see Figure 1A). Enhanced PTEN activity translated into the functional consequence of suppressed AKT activity after FcγR stimulation in BMT AMs, but we observed that AKT function could be restored with indomethacin pretreatment (see Figure 1B). Thus, these data suggest that PGE2 may increase BMT AM PTEN activity and diminish AKT activation after FcγR activation.
During acute bacterial lung infections, AM phagocytosis likely involves uptake of bacteria that are nonopsonized or opsonized by serum-derived complement (6, 43). It has previously been shown that PI3K activity, which is tightly regulated by PTEN, is involved in complement-mediated and nonopsonized phagocytosis (27–29) in nontransplant settings. Therefore, we examined whether inhibiting PTEN activity could restore BMT AM phagocytosis of serum and non–serum-opsonized FITC–P. aeruginosa. Pharmacologic PTEN inhibition increased phagocytosis of nonopsonized P. aeruginosa but did not restore levels to those seen in control AMs. In contrast, pharmacologic inhibition of PTEN fully restored serum-opsonized phagocytosis to control levels (see Figure 2). It is likely that the presence of IgG (in addition to complement) in our immune serum contributed to the greater magnitude of improvement we observed in phagocytosis of serum-opsonized bacteria in bpV(pic)-treated BMT AMs compared with nonopsonized bacteria. As discussed below, these pharmacologic results were corroborated by studies using genetic deletion of PTEN.
It is interesting to speculate on the role that PTEN activation may play during acute infection in control mice. Our data demonstrate that PTEN activity is low in control AMs, allowing AKT to be phosphorylated in response to FcγR signaling (see Figure 1). It is likely, however, that during the course of infection, inflammatory mediators (including PGE2) may up-regulate PTEN activity at later time points after infection. Up-regulation of PTEN activity at later time points may serve as a negative-feedback control mechanism to limit further macrophage activation via IgG-opsonized targets. This may prevent potentially harmful and prolonged activation of the innate immune response.
Previous studies have shown that in murine models of neutropenia, myeloid-specific ablation of PTEN can enhance opsonized bacterial clearance and proinflammatory cytokine production in AMs (38). Our data demonstrate a similar enhancement of host defense after transplantation of myeloid-specific PTEN KO bone marrow into WT recipients. Despite overproduction of PGE2 (see Figure 3D), we found that AM phagocytosis and killing of serum-opsonized P. aeruginosa is restored (see Figures 3B and 3C) and that AM TNF-α production is improved in the absence of PTEN expression after BMT (see Figure 4A). Phagocytosis of nonopsonized bacteria is not fully restored in PTEN CKO BMT AMs (see Figure 3E). Furthermore, bacterial clearance in PTEN CKO BMT mice challenged with P. aeruginosa lung infection is only partially recovered (see Figure 5), despite the fact that lung TNF-α levels are restored (see Figure 4B). Taken together, these data suggest that PTEN may be necessary for PGE2 to mediate its suppressive effects on BMT AM function against opsonized targets; however, BMT AMs retain PTEN-independent defects in nonopsonized phagocytosis.
After infection, total lung leukocyte numbers and neutrophil recruitment are similar in WT BMT and PTEN CKO BMT mice (Figures 7B and 7D). However, uninfected PTEN CKO BMT mice display an increase in total leukocyte number relative to WT BMT mice (Figure 7A). We found significantly more neutrophils and fewer macrophages in the lungs of uninfected PTEN CKO BMT mice compared with uninfected control and WT BMT mice (Figure 7C). In Figure 7E, we demonstrate that neutrophil killing of serum-opsonized P. aeruginosa is restored to control levels in PTEN CKO BMT mice. Taken together, our results indicate that PTEN CKO BMT mice have fully restored AM phagocytosis of serum-opsonized bacteria, more neutrophils at baseline, and normal neutrophil killing ability.
Despite these improvements in host defense, in vivo clearance of P. aeruginosa in PTEN CKO BMT mice is not fully restored to control levels. We believe this is best explained by the observation that nonopsonized bacterial phagocytosis is still impaired in PTEN CKO BMT AMs relative to control AMs (see Figure 3E). This is likely because IRAK-M is elevated in the AMs from PTEN CKO BMT mice (see Figure 6). This elevation in IRAK-M in the PTEN CKO BMT AMs may also explain why TNF-α production is not fully restored to control levels (see Figure 4A), assuming the signal to up-regulate TNF-α is mediated via TLR signaling. We have previously shown that increased PGE2 production after BMT elevates IRAK-M expression in AMs (20). Transplantation of IRAK-M−/− bone marrow into WT mice fully restores AM phagocytosis of nonopsonized P. aeruginosa and in vivo clearance of P. aeruginosa after an acute (24-h) infection. Putting our two studies together, we conclude that nonopsonized phagocytosis of P. aeruginosa by AMs plays a critical role in clearance of acute infection and is negatively regulated by PGE2–induced IRAK-M. Although we believe the partial restoration of bacterial clearance in our PTEN CKO BMT mice is best explained by our observation that nonopsonized phagocytosis by AMs is not fully recovered, another possibility is that neutrophils in the PTEN CKO BMT may display prolonged survival (30, 38), resulting in increased neutrophil damage to the lung, delayed immune resolution, and diminished tissue repair. Because PTEN is a critical regulator of cellular apoptosis, this may well be occurring.
IRAK-M protein expression is elevated in WT BMT and PTEN CKO BMT AMs relative to control AMs (see Figure 6). These data suggest that PGE2–mediated up-regulation of IRAK-M may occur independently of PTEN to regulate AM host defense functions. Thus, our data support a model where PGE2 signaling induces two unique pathways that regulate host defense. The up-regulation of PTEN by PGE2 primarily causes inhibition of opsonized AM phagocytosis and neutrophil killing after BMT. Simultaneously, PGE2–mediated up-regulation of IRAK-M inhibits nonopsonized phagocytosis in AMs after BMT.
Susceptibility to P. aeruginosa is widely considered to be a consequence of neutropenia (44). However, our data demonstrate that isolated defects in AM phagocytosis of nonopsonized bacteria can render the host susceptible to this infection. Our findings are supported by previous observations demonstrating that clodronate liposome depletion of AMs negatively affects lung host defense against P. aeruginosa in vivo (44).
Overall, our data suggest that PGE2 may inhibit multiple mechanisms of phagocytosis in BMT AMs via the up-regulation of PTEN activity and the induction of IRAK-M. Blocking PTEN activity mitigates the suppressive effects of PGE2 on opsonized bacterial phagocytosis in AMs and restores neutrophil killing after BMT. However, ablation of PTEN may not be sufficient to fully restore host defense against acute P. aeruginosa infection, given that nonopsonized phagocytosis by PTEN CKO BMT AMs appears to be inhibited by elevated IRAK-M expression. As such, our results suggest that a better strategy to restore host defense after BMT may be to target the production of PGE2 or EP2 receptor signaling. In fact, pharmacologic blockade of PGE2 production by indomethacin after BMT can fully restore host defense (12). An important future goal will be to determine whether these same PGE2–induced alterations are present in human AMs after HSCT.
Supplementary Material
Acknowledgments
The authors thank C. Henrique Serezani and Rommel Sagana for technical assistance with Western blot analysis.
Footnotes
This work was supported by National Institutes of Health grants AI065543 (B.B.M.) and HL085083 (E.S.W.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0079OC on April 28, 2011
References
- 1.Kotloff R, Ahya V, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48 [DOI] [PubMed] [Google Scholar]
- 2.Ballinger MN, McMillan TR, Moore BB. Eicosanoid regulation of pulmonary innate immunity post-hematopoietic stem cell transplantation. Arch Immunol Ther Exp (Warsz) 2007;55:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–1826 [DOI] [PubMed] [Google Scholar]
- 4.Auletta JJ, Lazarus HM. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant 2005;35:835–857 [DOI] [PubMed] [Google Scholar]
- 5.Guillaume T, Rubinstein DB, Symann M. Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood 1998;92:1471–1490 [PubMed] [Google Scholar]
- 6.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc 2005;2:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri N, Sabroe I. Basic science of the innate immune system and the lung. Paediatr Respir Rev 2008;9:236–242 [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev 2000;173:39–51 [DOI] [PubMed] [Google Scholar]
- 9.Winston DJ, Territo MC, Ho WG, Miller MJ, Gale RP, Golde DW. Alveolar macrophage dysfunction in human bone marrow transplant recipients. Am J Med 1982;73:859–866 [DOI] [PubMed] [Google Scholar]
- 10.Zimmerli W, Zarth A, Gratwohl A, Speck B. Neutrophil function and pyogenic infections in bone marrow transplant recipients. Blood 1991;77:393–399 [PubMed] [Google Scholar]
- 11.Ojielo C, Cooke KR, Mancuso P, Standiford TJ, Olkiewicz KM, Cloutheir S, Corrion L, Ballinger MN, Toews GB, Paine R, et al. Defective phagocytosis and clearance of pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol 2003;171:4416–4424 [DOI] [PubMed] [Google Scholar]
- 12.Ballinger MN, Aronoff DM, McMillan TR, Cooke KR, Okiewicz K, Toews GB, Peters-Golden M, Moore BB. Critical role of prostaglandin e2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol 2006;177:5499–5508 [DOI] [PubMed] [Google Scholar]
- 13.Hubbard LL, Ballinger MN, Wilke CA, Moore BB. Comparison of conditioning regimens for alveolar macrophage reconstitution and innate immune function post bone marrow transplant. Exp Lung Res 2008;34:263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin e2 inhibits alveolar macrophage phagocytosis through an e-prostanoid 2 receptor-mediated increase in intracellular cyclic amp. J Immunol 2004;173:559–565 [DOI] [PubMed] [Google Scholar]
- 15.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic amp (camp): differential roles of protein kinase a and exchange protein directly activated by camp-1. J Immunol 2005;174:595–599 [DOI] [PubMed] [Google Scholar]
- 16.Armstrong RA. Investigation of the inhibitory effects of pge2 and selective ep agonists on chemotaxis of human neutrophils. Br J Pharmacol 1995;116:2903–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel SL, Spengler M, May MA, Spengler R, Larrick J, Remick D. Prostaglandin e2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem 1988;263:5380–5384 [PubMed] [Google Scholar]
- 18.Caggiano AO, Kraig RP. Prostaglandin e receptor subtypes in cultured rat microglia and their role in reducing lipopolysaccharide-induced interleukin-1beta production. J Neurochem 1999;72:565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki K, Noguchi K, Endo H, Kondo H, Ishikawa I. Prostaglandin e2 downregulates interleukin-12 production through ep4 receptors in human monocytes stimulated with lipopolysaccharide from actinobacillus actinomycetemcomitans and interferon-gamma. Oral Microbiol Immunol 2003;18:150–155 [DOI] [PubMed] [Google Scholar]
- 20.Hubbard LL, Ballinger MN, Thomas PE, Wilke CA, Standiford TJ, Kobayashi KS, Flavell RA, Moore BB. A role for il-1 receptor-associated kinase-m in prostaglandin e2-induced immunosuppression post-bone marrow transplantation. J Immunol 2010;184:6299–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canetti C, Serezani CH, Atrasz RG, White ES, Aronoff DM, Peters-Golden M. Activation of phosphatase and tensin homolog on chromosome 10 mediates the inhibition of fcgammar phagocytosis by prostaglandin e2 in alveolar macrophages. J Immunol 2007;179:8350–8356 [DOI] [PubMed] [Google Scholar]
- 22.Gunzl P, Schabbauer G. Recent advances in the genetic analysis of pten and pi3k innate immune properties. Immunobiology 2008;213:759–765 [DOI] [PubMed] [Google Scholar]
- 23.Korade-Mirnics Z, Corey SJ. Src kinase-mediated signaling in leukocytes. J Leukoc Biol 2000;68:603–613 [PubMed] [Google Scholar]
- 24.Swanson JA, Hoppe AD. The coordination of signaling during fc receptor-mediated phagocytosis. J Leukoc Biol 2004;76:1093–1103 [DOI] [PubMed] [Google Scholar]
- 25.Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding pten regulation: Pip2, polarity and protein stability. Oncogene 2008;27:5464–5476 [DOI] [PubMed] [Google Scholar]
- 26.White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, Moore BB, Peters-Golden M. Prostaglandin e(2) inhibits fibroblast migration by e-prostanoid 2 receptor-mediated increase in pten activity. Am J Respir Cell Mol Biol 2005;32:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulahian TH, Imrich A, Deloid G, Winkler AR, Kobzik L. Signaling pathways required for macrophage scavenger receptor-mediated phagocytosis: analysis by scanning cytometry. Respir Res 2008;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox D, Dale BM, Kashiwada M, Helgason CD, Greenberg S. A regulatory role for src homology 2 domain-containing inositol 5′-phosphatase (ship) in phagocytosis mediated by fc gamma receptors and complement receptor 3 (alpha(m)beta(2); cd11b/cd18). J Exp Med 2001;193:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vachon E, Martin R, Plumb J, Kwok V, Vandivier RW, Glogauer M, Kapus A, Wang X, Chow CW, Grinstein S, et al. Cd44 is a phagocytic receptor. Blood 2006;107:4149–4158 [DOI] [PubMed] [Google Scholar]
- 30.Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, You J, Le Y, Honczarenko M, Silberstein L, et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA 2006;103:14836–14841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballinger MN, Hubbard LL, McMillan TR, Toews GB, Peters-Golden M, Paine R, III, Moore BB. Paradoxical role of alveolar macrophage-derived granulocyte-macrophage colony-stimulating factor in pulmonary host defense post-bone marrow transplantation. Am J Physiol Lung Cell Mol Physiol 2008;295:L114–L122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-lipoxygenase reaction products modulate alveolar macrophage phagocytosis of klebsiella pneumoniae. Infect Immun 1998;66:5140–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol 1996;135:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against klebsiella pneumoniae through the activation of nadph oxidase. Blood 2005;106:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med 2003;168:436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao X, Wei G, Fang H, Guo J, Weinstein M, Marsh CB, Ostrowski MC, Tridandapani S. The inositol 3-phosphatase pten negatively regulates fc gamma receptor signaling, but supports toll-like receptor 4 signaling in murine peritoneal macrophages. J Immunol 2004;172:4851–4857 [DOI] [PubMed] [Google Scholar]
- 37.Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent pten inhibitors. FEBS Lett 2004;566:35–38 [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, You J, Robson BE, Umetsu DT, Mizgerd JP, et al. Targeted deletion of tumor suppressor pten augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood 2009;113:4930–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leenen PJ, Canono BP, Drevets DA, Voerman JS, Campbell PA. Tnf-alpha and ifn-gamma stimulate a macrophage precursor cell line to kill listeria monocytogenes in a nitric oxide-independent manner. J Immunol 1994;153:5141–5147 [PubMed] [Google Scholar]
- 40.Munoz-Fernandez MA, Fernandez MA, Fresno M. Activation of human macrophages for the killing of intracellular trypanosoma cruzi by tnf-alpha and ifn-gamma through a nitric oxide-dependent mechanism. Immunol Lett 1992;33:35–40 [DOI] [PubMed] [Google Scholar]
- 41.Munoz-Fernandez MA, Fernandez MA, Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol 1992;22:301–307 [DOI] [PubMed] [Google Scholar]
- 42.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor pten is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood 2007;109:4028–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palecanda A, Kobzik L. Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr Mol Med 2001;1:589–595 [DOI] [PubMed] [Google Scholar]
- 44.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in pseudomonas aeruginosa pneumonia. Infect Immun 1998;66:3164–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.