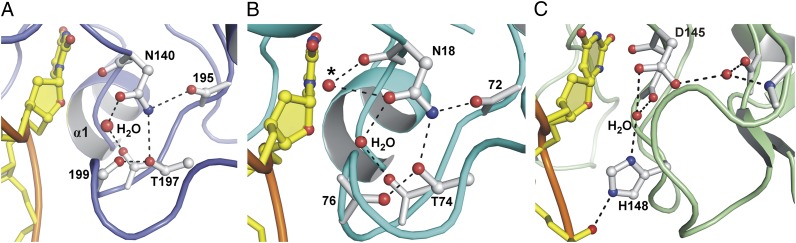
Fig. 2.
Coordination of the water nucleophile in TDG-MUG enzymes. (A) Close-up view of the TDG active site shows that the putative water nucleophile is coordinated by the Asn140 side chain oxygen (Oδ1) and the backbone oxygen of Thr197. Also shown are interactions that position the Asn140 side chain, and contacts with the Thr197 hydroxyl side chain. (B) A revised mechanism for nucleophile coordination is proposed for MUG enzymes, involving the Asn18 side chain Oδ1 and the Thr74 backbone oxygen (E. coli MUG numbering). The previous proposal that MUG coordinates the nucleophile using the backbone oxygen and side chain Oδ1 of Asn18 (33) results in substantially different position for the nucleophile (marked by *). Note that a nucleophile is not observed in the eMUG E·S complex (PDB ID: 1MWJ), but two water molecules contact Asn18 in the free enzyme (PDB ID: 1MUG). The figure shows free MUG (with both water molecules) and DNA from the MUG E·S complex (proteins aligned). (C) A similar view of the E·S complex for human UNG (PDB ID: 1EMH) (31) shows that the position of the nucleophile relative to the electrophile (C1′) is very similar to that observed here for TDG (and proposed for eMUG).