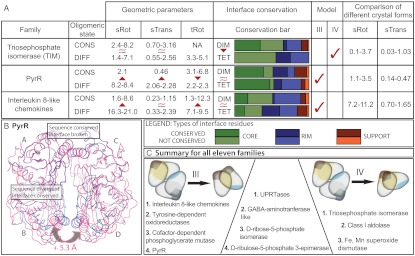
Fig. 4.
Conservation of interfaces and geometric changes. (A) Summary of geometric and sequence comparison parameters for three families. The table provides ranges of values for geometric parameters (sRot, sTrans and tRot) as well as proportions of conserved and nonconserved residues in both interfaces for all pairs with different oligomeric states within a family. Interface core, rim, and support residues are defined as in ref. 7. Higher geometric variation between homologues with different (DIFF) oligomeric state than the ones with conserved (CONS) oligomeric state indicates the geometric (III) evolutionary model. Lower sequence conservation of the tetrameric than the dimeric interface between pairs of homologues with different oligomeric state indicates the direct (IV) evolutionary model. (B) A hypothetical tetramer of B. subtilis PyrR (PDB ID code 1A3C, magenta) is constructed by superposition on to B. caldolyticus PyrR (PDB ID code 1NON, blue), showing evolutionary change in subunit orientation and the relative positions of the two interfaces. Because the residues forming the BcPyrR tetrameric interface helix are completely conserved in sequence between the two species, the 5.3-Å increase in distance between subunits B and D cannot be a matter of local change in the interface but rather due to the difference in complex geometry. (C) Summary for all 11 families. Comparison of homologues reveals high interdependence of oligomeric state and complex geometry (geometric model III) in four protein families. In three families the evolutionary change in oligomeric state is predominantly driven by sequence changes in the interface (direct model IV). The remaining four families represent an evolutionary hybrid that encompasses elements of both the geometric and the direct model.