Abstract
It is well established that estradiol (E2) decreases food intake and body weight in young female rats. However, it is not clear if female rats retain responsiveness to the anorexigenic effect of E2 during middle age. Because middle-aged females exhibit reduced responsiveness to E2, manifesting as a delayed and attenuated luteinizing hormone surge, it is plausible that middle-aged rats are less responsive to the anorexigenic effect of E2. To test this we monitored food intake in ovariohysterectomized young and middle-aged rats following E2 treatment. E2 decreased food intake and body weight to a similar degree in both young and middle-aged rats. Next, we investigated whether genes that mediate the estrogenic inhibition of food intake are similarly responsive to E2 by measuring gene expression of the anorexigenic genes corticotropin-releasing hormone (CRH), proopiomelanocortin (POMC), the long form of the leptin receptor (Lepr) and serotonin 2C receptors (5HT2CR) and the orexigenic genes agouti-related peptide (AgRP), neuropeptide Y (NPY), prepromelanin-concentrating hormone (pMCH) and orexin in the hypothalamus of young and middle-aged OVX rats treated with E2. As expected, E2 increased expression of all anorexigenic genes while decreasing expression of all orexigenic genes in young rats. Although CRH, 5HT2CR, Lepr, AgRP, NPY and orexin were also sensitive to E2 treatment in middle-aged rats, POMC and pMCH expression were not influenced by E2 in middle-aged rats. These data demonstrate that young and middle-aged rats are similarly sensitive to the anorexigenic effect of E2 and that most, but not all feeding-related genes retain sensitivity to E2.
Keywords: Estradiol, Estrogen Receptor Alpha, Food Intake, Aging
INTRODUCTION
The hypothalamus is a key regulator of a number of homeostatic processes, one of which is the regulation of feeding behavior. In female mammals, estradiol (E2) acts within the hypothalamus to inhibit food intake [1–3]. In cycling rats, food intake is lowest on the day of estrus [4–6]. Daily food intake increases following removal of endogenous E2 via ovariectomy [7], which can be prevented by E2 replacement [8, 9]. Moreover, site specific infusion of E2 into the arcuate nucleus (ARC) and medial preoptic area (MPOA) of the hypothalamus decreases food intake [2, 3]. E2 appears to inhibit food intake through nuclear estrogen receptors (ERs) to increase anorexigenic signaling mediated by peptides such as corticotropin releasing hormone (CRH) [10], and by decreasing orexigenic signals, such as neuropeptide Y (NPY) [11], in the hypothalamus. Although there are two nuclear ERs, ERα and ERβ, E2’s inhibitory effect on feeding appears to be mediated solely through ERα. ERα knockout mice (αERKO) have increased body weight and food intake compared to wild-type controls [12] and following ovariectomy, E2 treatment does not decrease food intake [13]. In addition, pharmacological studies demonstrated that an ERα but not an ERβ agonist decreases food intake in rats and mice [14–16] and that blockade of ERα but not ERβ with selective antagonists prevents the inhibitory effect of endogenous and exogenous E2 on food intake [17].
E2 also acts within the hypothalamus as an important regulator of the luteinizing hormone (LH) surge. E2 exerts positive feedback actions on gonadotropin releasing hormone (GnRH) neurons, stimulating GnRH release into the hypophysial portal blood system to affect pituitary gonadotropes. The subsequent secretion of LH from the pituitary triggers ovulation [18]. This appears to be mediated by ERα as αERKO mice are infertile due in part to a disruption in the LH surge [19]. Although GnRH neurons do not express ERα [20], E2 regulates these neurons indirectly through afferent inputs from other E2-responsive neurons within the hypothalamus, such as the neurons synthesizing the peptide kisspeptin, along with neurons that release the neurotransmitters GABA, glutamate and norepinephrine [21–24]. Interestingly, as females rodents enter middle age, multiple E2-responsive neurons within the hypothalamus become less sensitive to E2 positive feedback, which results in a delayed and attenuated LH surge [25, 26].
The menopausal transition is associated with increased morbidity that negatively affects the quality of life [26]. In particular, increased intra-abdominal fat during the perimenopausal years is associated with an elevated risk of metabolic and cardiovascular diseases [27, 28]. Therefore, understanding the interaction of age and hormones on the capacity of the neuroendocrine axis to regulate eating behavior and /or maintain energy balance in middle-aged females may reveal potential interventions that reduce morbidity and improve quality of life. Although it is well known that aging impairs E2’s ability to induce the LH surge [25, 26, 29], the effect of age on E2’s anorexigenic properties, which are also mediated by the neuroendocrine axis, have not been investigated. Because both the LH surge and E2’s anorexigenic effect are mediated by ERα in the hypothalamus, it is possible that the anorexigenic effect of E2 is also affected by reproductive aging. Here we tested the hypothesis that E2’s anorexigenic effect is attenuated in middle-aged compared to young female rats. We also determined whether feeding related genes in the hypothalamus are similarly regulated by E2 in young and middle-aged rats.
MATERIALS AND METHODS
Animals and Ovariohysterectomy
Young (2 month) and middle-aged (retired breeders, 8–12 month) Sprague-Dawley female rats (Charles Rivers, Wilmington, MA) were group housed in standard cages with free access to food and water, except where otherwise noted. Other labs have reported no differences in hypothalamic responsiveness to E2 between middle-aged virgin and retired breeders [30–32]; therefore, this study used retired breeders. Room temperature was maintained at 20 ± 2°C with a 14:10 light cycle (lights off at 10 pm). Rats were anesthetized with intraperitoneal injections of a mixture of ketamine (80 mg/kg) and xylazine (4.6 mg/kg) and then bilaterally ovariohysterectomized (OVX) using an intra-abdominal approach. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Rats and were approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine.
Experiment 1: Anorexigenic effect of estradiol in young and middle-aged rats
Three days after OVX, six young and six middle-aged rats were transferred to individual test cages designed to continually measure food intake (Med Associates, Georgia, VT). The test cages were equipped with a water spout and a food trough, a 45-mg chow pellet dispenser that used a photobeam to detect the presence of a pellet. Food and water were continuously available except during a daily 1 h period (0900–1000 h) when rats were weighed and food dispensers and water bottles were refilled. Each testing cage was enclosed in an outer box with a 12:12 h light cycle (lights off at 1300 h) and equipped with a white noise generator.
Following a seven day adaptation period, body weight and 24 h food intake were measured daily between 0900 and 1000 h for a four day testing period. All rats received a single subcutaneous injection of 2 μg E2 benzoate (Steraloids, Inc., Newport, RI) dissolved in 0.1 ml peanut oil 4 h before the dark phase on day 3. This schedule of hormone treatment and four-day behavioral assessments were based on a previous report that an acute injection of E2 on day 3 of a 4 day cycle models the changes in E2 secretion observed across the estrous cycle and decreases food intake on day 4 (the day that represents behavioral estrus) in young rats [9].
Experiment 2: Influence of E2 on anorexigenic and orexigenic gene young and middle-aged rats
Seven days after OVX, six young and six middle-aged rats were injected with either vehicle or 2 μg E2 in 0.1 ml peanut oil for two consecutive days at 0900 h. The two injection E2 paradigm was used to be consistent with previous studies examining E2 regulation of orexigenic and anorexigenic gene expression [33] and because this type of E2 treatment more closely models the short-term fluctuations of a natural estrous cycle. Additionally, similar changes are reported when acute and chronic E2 treatment is used. For example, both acute and chronic E2 treatment decreases MCH gene expression in the hypothalamus [34, 35]. In the afternoon on the third day (1300 h) rats were anesthetized with an overdose of ketamine and xylazine and then decapitated. The anterior hypothalamus, which included the MPOA, and the posterior hypothalamus, which included the ARC, paraventricular nucleus (PVN) and the lateral hypothalamus (LH), were dissected as previously described [36], frozen on dry ice and stored at −80°C. CRH mRNA was analyzed in the anterior hypothalamus, and proopiomelanocortin (POMC), the long form of the leptin receptor (Lepr) and serotonin 2C receptors (5HT2CR) and the orexigenic peptides agouti-related peptide (AgRP), NPY, prepromelanin-concentrating hormone (pMCH) and orexin mRNAs were analyzed in the posterior hypothalamus.
cDNA Synthesis and RT-PCR
Real-time PCR was used to quantify mRNA levels in hypothalamic samples. DNA-free total RNA was purified using the RNeasy lipid minikit (QIAGEN, Valencia, CA), including a deoxyribonuclease step. Reverse transcription (RT) was performed with 500 ng of RNA using the high-capacity cDNA RT kit with ribonucleaseinhibitor (Applied Biosystems, Foster City, CA). Real-time PCR was carried out using SYBR GREEN gene master mix (AppliedBiosystems) according to the manufacturer’s instructions. The primer sequences are listed in Table 1.
Table 1.
Primer Sequences Used for RT-PCR
| Gene | Sense | Antisense |
|---|---|---|
| CRH | 5’GCT GTC CCC CAA CTC CAC | 5’ CAG CTC CGT GCT GCT GTC |
| POMC | 5’ CTC CAT AGA CGT GTG GAG CTG | 5’ TCA GTC AAG GGC TGT TCA TCT |
| Lepr | 5’AGG GAA CCT GTG AGG ATG AGT | 5’ TGT CTC AGT GGG GAA TGT TTC |
| 5HT2CR | 5’ AGA AAG AAA AGC GTC CCA GAG | 5’CCA CAA AGA ACC GAC AGG ATA |
| AgRP | 5’TGA AGA AGA CAG CAGCAG ACC | 5’AAG GTA CCT GTT GTC CCA AGC |
| NPY | 5’GTA ACA AAC GAA TGG GGC TGT | 5’ CGC AGA GCG GAG TAG TAT CTG |
| pMCH | 5’ ATG CTG GCC TTT TCT TTG TTT | 5’ AAG GAG CAA CAA CCG ATC TTT |
| Orexin | 5’GCA TCC TCA CTC TGG GAA AG | 5’ GCA GGG ATA TGG CTC TAG CTC |
| β-actin | 5’AGA TTA CTG CCC TGG CTC CTA | 5’CTC ATC GTA CTC CTG CTT GCT |
Abbreviations: CRH = corticotropin releasing hormone; POMC = proopiomelanocortin; Lepr = long form of the leptin receptor; 5HT2CR = serotonin 2C receptor; AgRP = agouti-related peptide; NPY = neuropeptide Y; pMCH = prepromelanin-concentrating hormone.
Data Analysis
Data are expressed as means ± SEM throughout. Due to the significant difference in body weight between the young and middle-aged rats (middle-aged>young, F = 33.7, P < 0.05), food intake was normalized to 100 g body weight. A mixed design ANOVA (within factor: hormone; between factor: age) was used to compare the anorexigenic effect of E2 in young and middle-aged rats. The anorexigenic effect of E2 was assessed by comparing food intake and body weight on day 2 (the day that represents diestrus 2) and day 4 (the day that represents estrus) [15]. Additionally, the effect of E2 on the percent change in body weight from day 2 to day 4 in young and middle-aged rats was analyzed via an independent measures t-test. RT-PCR values were calculated using the ΔΔCT quantification method using β-actin as the normalizing housekeeping gene. Two factor ANOVAs (hormone by age) were used to determine differences in mRNA expression. Bonferroni post hocs were used throughout to determine individual group differences following significant main or interaction ANOVA effects (P< 0.05).
RESULTS
Experiment 1
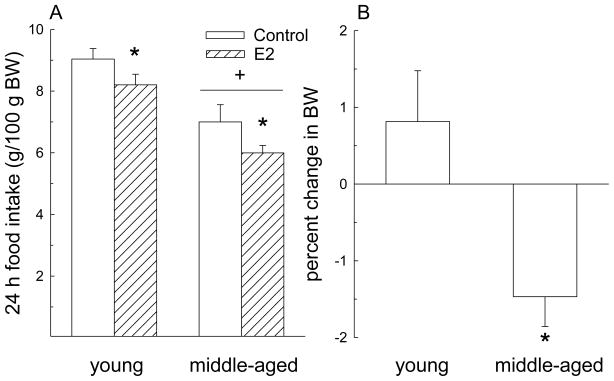
Both age and E2 had significant effects on food intake (Fig. 1A). Following E2 injection on day 3, food intake on day 4 (the day that models estrus) decreased compared to food intake on day 2 (the day that models diestrus 2; F = 17.5, P< 0.01). Regardless of hormone treatment, middle-aged rats consumed less food than young rats (F = 17.8, P< 0.01). However, the magnitude of decrease in food intake following hormone treatment was similar in young and middle-aged rats (hormone × age interaction, F = 0.14, n.s.). The change in body weight following hormone treatment was different between young and middle-aged rats (t = 2.97, P< 0.05; Fig. 1B). Middle-aged rats lost ~1.4% of their body weight compared to a gain of 0.8% in young rats. This difference is likely the result of the different growth curves in young and middle-aged rats [37]. An additional analysis revealed an interaction between E2 and age on body weight (F = 12.5, P < 0.05). E2 treatment decreased body weight in middle-aged rats (P < 0.05) but not in young rats. However, prior to hormone treatment young rats gained ~6 g per day, while middle-aged rats gained ~ 2 g per day. Therefore, in two days one would expect a 12 g increase in body weight in young rats and a 4 g increase in body weight in middle-aged rats. Following E2 treatment, young rats only gained ~ 2 g while middle-aged rats lost ~ 6 g, which translates into a loss of ~10 g body weight for both age groups.
Figure 1.
E2 effects on food intake and body weight in young and middle-aged rats. (A) E2 treatment decreased 24 h food intake in both young and middle-aged rats. Regardless of hormone treatment, 24 h food intake was lower in middle-aged than in young rats. *< control, P< 0.05. +< young, P< 0.05. (B) The percent change in body weight following E2 treatment was different in young and middle-aged rats. Young rats gained ~0.8% whereas middle-aged rats lost ~1.4% of their body weight. Data are expressed as mean ± SEM. *< young, P< 0.05.
Experiment 2
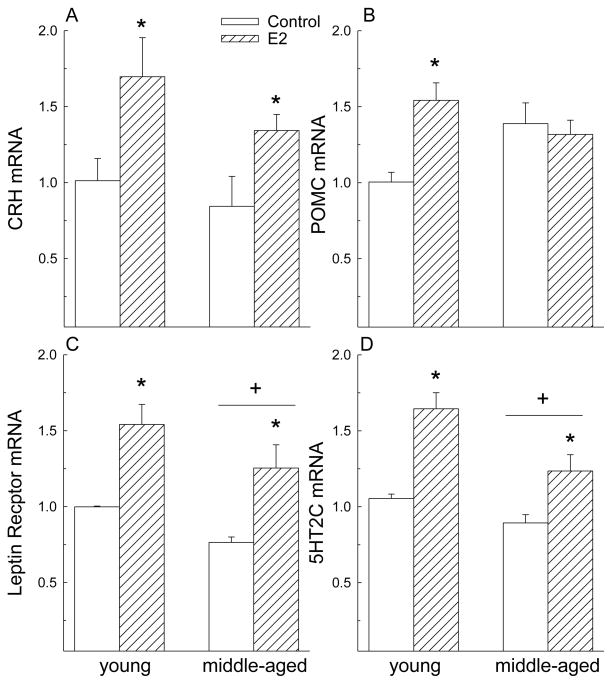
As expected, E2 increased expression of anorexigenic genes in the hypothalamus (Fig. 2). Regardless of age, E2 increased expression of CRH mRNA (F = 10.3, P< 0.01) in the anterior hypothalamus and of Lepr (F = 25.2, P< 0.01) and 5HT2CR (F = 32.8, P < 0.01) mRNA levels in the posterior hypothalamus. Regardless of hormone treatment, expression of Lepr (F = 6.4, P< 0.05) and 5HT2CR (F = 12.3, P< 0.01) mRNA was lower in middle-aged compared to young rats. There was no interaction between hormone and age on CRH (F = 0.25, n.s), Lepr (F = 0.06, n.s.), nor 5HT2CR (F = 2.3, n.s.) expression. However, there was an interaction between E2 and age on expression of POMC in the posterior hypothalamus (F = 8.27, P< 0.05). Although POMC expression increased in young rats following E2 treatment, this effect was absent in middle-aged rats.
Figure 2.
E2 increased expression of most anorexigenic genes in the hypothalamus. (A) E2 increased expression of CRH in the anterior hypothalamus of young and middle-aged rats. (B) E2 increased expression of POMC in the posterior hypothalamus of young but not middle-aged rats. (C, D) E2 increased expression of the leptin and 5HT2C receptors in the posterior hypothalamus of young and middle-aged rats. Regardless of hormone, leptin and 5HT2C receptor expression was lower in middle-aged than in young rats. Data are expressed as mean ± SEM. RT-PRC values are expressed as fold change relative to young, oil-treated control. *>control, P< 0.05.+< young, P< 0.05.
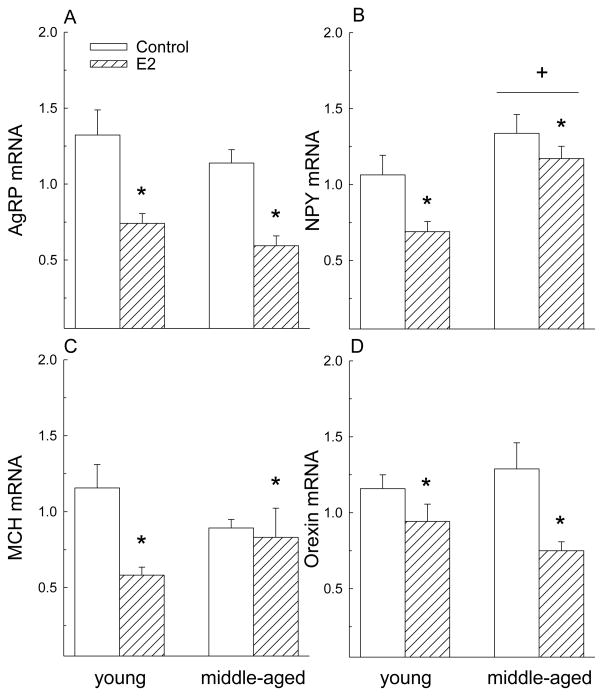
As expected, E2 decreased expression of orexigenic genes in the posterior hypothalamus (Fig. 3). Regardless of age, expression of AgRP (F = 29.4, P< 0.01), NPY (F = 6.8, P< 0.05), pMCH (F = 6.1, P< 0.05), and orexin (F = 10.5, P< 0.01) decreased following E2 treatment. Although there was a significant main effect of hormone on pMCH expression (F = 6.1, P < 0.05), this is due primarily to the very large decrease in young rats as a priori post hoc tests demonstrated a reduction in pMCH expression in young (P< 0.05) but not middle-aged rats (P> 0.05). Additionally, regardless of hormone treatment, young rats had lower NPY mRNA levels than middle-aged rats (F = 13.3, P< 0.01).
Figure 3.
E2 decreased expression of orexigenic genes in the posterior hypothalamus. (A) E2 decreased expression of AgRP in young and middle-aged rats. (B) E2 decreased expression of NPY in young and middle-aged rats. Regardless of hormone, NPY expression was higher in middle-aged than in young rats. (C, D) There was a main effect of hormone on MCH and orexin expression. E2 decreased expression of MCH and orexin in young and middle-aged rats. Data are expressed as mean ± SEM. RT-PRC values are expressed as fold change relative to young, oil-treated control *<control, P< 0.05. +> young, P< 0.05.
DISCUSSION
This study tested the hypothesis that middle-aged female rats, which show delayed and attenuated E2- dependent LH surges, are less sensitive to the anorexigenic effect of exogenous E2 and determined whether E2 regulates feeding-related genes in the hypothalamus of middle-aged females. Our results demonstrate that the anorexigenic effect of E2 is similar in middle-aged and young females; both age groups showed a comparable suppression in food intake and body weight gain following E2 treatment. We also found that most genes that mediate E2’s anorexigenic effect retain sensitivity to E2 regulation in the hypothalamus of middle-aged rats. These effects of E2 are likely mediated by ERα as it is the nuclear receptor that is both necessary and sufficient for E2’s inhibitory effect on food intake [14–17]. It is interesting to note that this is in contrast with another ERα mediated response in the hypothalamus, most notably positive feedback regulation of the LH surge, which is impaired by aging [25, 26].
Because the anorexigenic effect of E2 was unaffected by age, we tested the hypothesis that E2-sensitive, feeding related genes in the hypothalamus are regulated similarly by E2 in young and middle-aged rats. We tested 4 anorexigenic genes whose mRNA levels in the hypothalamus increase in response to E2 and may be involved in mediating E2’s inhibitory effect on food intake. CRH in the MPOA is an important mediator of E2’s inhibitory effect on food intake. CRH and ERα are co-expressed in MPOA neurons [10], E2 treatment increases CRH expression in young OVX rats [38], and central treatment with a CRH antagonist blocks E2’s anorexigenic effect in young OVX rats [10]. In addition, site specific infusion of E2 into the MPOA decreases 24 h food intake in young OVX rats [2, 3]. The ARC is another hypothalamic site where E2 infusion decreases 24 h food intake [3]. POMC neurons are abundant within the ARC, and E2 treatment increases POMC mRNA expression in young OVX mice [39]. Leprs are also expressed throughout the hypothalamus, with particularly high expression in the ARC where they are co-expressed with ERs [40]. In young rats long-term OVX (22 weeks) decreases hypothalamic Lepr expression, and E2 treatment throughout this period prevents the decrease [41]. A shorter period of E2 replacement (8 days) also increases Lepr expression in young OVX rats [42]. Additionally, central and peripheral E2 treatment increases leptin’s anorexigenic effect in young OVX rats [43]. Finally, in young OVX rats, a 5HT2C receptor agonist increases E2’s inhibitory effect on food intake [44]. As expected, E2 treatment increased expression of CRH, POMC, Lepr and 5HT2CR mRNAs in young rats. E2 also increased expression of CRH, Lepr and 5HT2CR mRNA in middle-aged rats, but POMC expression did not increase in middle-aged females. This could suggest that CRH, Lepr and 5HT2CR are of particular importance in mediating E2’s anorexignic effect in both young and middle-aged rats whereas POMC neurons serve a lesser role. This is supported by Geary and colleagues who reported that in young OVX rats E2 does not influence the inhibitory effect of MTII, a synthetic melanocortin 3/4 agonist [45]. These observations suggest that E2’s inhibitory effect on food intake is not mediated by α-MSH, a functional derivative of POMC neurons.
We also examined expression of 4 orexigenic genes in the hypothalamus that are decreased by E2 treatment or likely involved in mediating E2’s inhibitory effect on food intake. In young rats, AgRP and NPY gene expression in the ARC increases after OVX [46]; both decrease in vitro and in vivo following E2 treatment [11, 39, 47] and E2 decreases NPY-induced feeding [48]. pMCH gene expression decreases in young OVX female and male rats following E2 treatment [34, 35], and in young rats endogenous and exogenous E2 decrease MCH-induced feeding [49]. Finally, in young OVX rats, E2 treatment decreases orexin mRNA expression in the hypothalamus [42]. As expected, E2 decreased expression of AgRP, NPY, pMCH and orexin mRNA in young rats. In middle-aged rats AgRP, NPY and orexin neurons were still sensitive to E2 as indicated by a decrease in mRNA expression following E2 treatment. However, E2 did not alter pMCH mRNA in middle-aged rats. These results suggest that AgRP, NPY and orexin, but not MCH, may be of particular importance in mediating E2’s inhibitory effect on food intake, especially in middle-aged rats. Interestingly, MCH neurons, similar to GnRH neurons, do not express ERα, suggesting that any influence of E2 on MCH-induced feeding is through afferent inputs, which could also be influenced by aging [50].
It is well known that ERα expressed within hypothalamic neurons is the crucial mediator of E2’s inhibition of feeding and its positive feedback effects on the LH surge. However, E2 regulation of the LH surge is impaired with aging, as middle-aged female rats have a delayed and attenuated LH surge [25, 26]. Accumulating evidence suggests that LH surge dysfunction reflects reduced sensitivity of specific hypothalamic neurons to E2 in middle-aged females. For example, kisspeptin neurons in the anteroventral periventricular region of the hypothalamus (AVPV) provide potent excitatory input to GnRH neurons [24]. In young rats E2 treatment increases the number of kisspeptin immunoreactive neurons in the AVPV. In middle-aged rats, the E2-induced increase in kisspeptin-positive neurons decreases markedly compared to young rats [51], suggesting that neurons involved in mediating the LH surge lose sensitivity to E2 with aging. Again, this is in contrast with these results, which demonstrate that most feeding related genes are regulated similarly by E2 in both young and middle-aged rats. Interestingly, we found that POMC and MCH mRNAs were not responsive to E2 treatment in middle-aged rats. Clegg and colleagues recently demonstrated that selective deletion of ERα in POMC neurons produced both hyperphagia and impaired fertility in female mice through inhibition of E2 negative feedback [52]. Therefore, although POMC is important for both feeding and reproduction, our results may suggest that POMC is more important for E2 regulation of gonadotropin release than of feeding.
Independent of hormone treatment, we observed age related decreases in Lepr and 5HT2CR mRNA within the hypothalamus. This extends reports that show less Lepr expression in aged male rats [53]. This likely contributes to decreased leptin signaling in aged animals. We are unaware of studies examining the expression of 5HT2CR in the hypothalamus in aged male or female rats. Because 5HT is involved in many behaviors, the reduced receptor expression could have a wide range of implications. We also observed that hypothalamic NPY mRNA expression increases in middle-aged compared to young rats. This finding is consistent with previous studies demonstrating that hypothalamic NPY gene expression increases in postmenopausal compared to premenopausal women [54] and that on the morning of proestrus NPY mRNA in the median eminence is higher in middle-aged compared to young rats [55]. This effect appears to sexually dimorphic in that male rats express less NPY with aging [56]. Additional studies will be necessary to understand the effect of sex and age on NPY mRNA expression and the functional consequence of age-related changes in NPY mRNA expression.
In conclusion, the hypothalamus is the major site of E2 regulation of food intake and reproduction. Here we demonstrate that E2’s inhibitory effect on feeding behavior and on most genes that mediate E2’s anorexigenic effect remain sensitive to E2 in middle-aged rats. Future studies will be necessary to determine if the observed changes in hypothalamic gene expression following hormone treatment translate into comparable changes in hypothalamic protein expression in young and middle-aged rats. Our results here contrast with the loss of sensitivity to E2 positive feedback regulation of the LH surge in middle-aged rats. Although the underlying mechanisms are unclear, because there is little evidence that levels of ERα in hypothalamic nuclei differ between young and middle-aged female rats [57], it is interesting to speculate that ERα function is influenced by aging in some but not all hypothalamic neuronal populations. Recently, Brann and colleagues demonstrated that in hippocampal but not uterine tissue of middle-aged rats, ERα protein levels decrease through proteasomal degradation of ERs through enhanced interaction with E3 ubiquitin ligase C terminus of heat shock cognate protein 70 interacting protein [58]. It is possible that in middle-aged rats ERα protein decreases in certain hypothalamic neuronal populations involved in mediating the LH surge but not in those mediating feeding behavior. Future studies are needed to test this hypothesis.
Research Highlights.
E2 decreases food intake to a similar degree in young and middle-aged female rats.
E2 increased expression of CRH, Lepr and 5HT2CR in young and middle-aged females.
However, E2 increased expression of POMC in young, but not in middle-aged females.
E2 decreased expression of NPY, AgRP and orexin in young and middle-aged females.
However, E2 decreased expression of pMCH in young, but not in middle-aged females.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD058155 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and by NIH grants P60 DK020541 and T32 AG023475 and the Department of Obstetrics and Gynecology and Women’s Health, Albert Einstein College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain research. 1984;322:41–8. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- 2.Dagnault A, Richard D. Involvement of the medial preoptic area in the anorectic action of estrogens. The American journal of physiology. 1997;272:R311–7. doi: 10.1152/ajpregu.1997.272.1.R311. [DOI] [PubMed] [Google Scholar]
- 3.Santollo J, Torregrossa AM, Eckel LA. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Hormones and behavior. 2011;60:86–93. doi: 10.1016/j.yhbeh.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewett RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Animal behaviour. 1973;21:772–80. doi: 10.1016/s0003-3472(73)80103-4. [DOI] [PubMed] [Google Scholar]
- 5.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiology & behavior. 1976;17:201–8. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 6.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiology & behavior. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 7.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. Journal of comparative and physiological psychology. 1975;88:183–93. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 8.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiology & behavior. 1994;56:281–9. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 9.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Hormones and behavior. 2002;42:461–71. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 10.Dagnault A, Ouerghi D, Richard D. Treatment with alpha-helical-CRF(9–41) prevents the anorectic effect of 17-beta-estradiol. Brain research bulletin. 1993;32:689–92. doi: 10.1016/0361-9230(93)90175-b. [DOI] [PubMed] [Google Scholar]
- 11.Baskin DG, Norwood BJ, Schwartz MW, Koerker DJ. Estradiol inhibits the increase of hypothalamic neuropeptide Y messenger ribonucleic acid expression induced by weight loss in ovariectomized rats. Endocrinology. 1995;136:5547–54. doi: 10.1210/endo.136.12.7588307. [DOI] [PubMed] [Google Scholar]
- 12.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–7. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 14.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiology & behavior. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293:R2194–201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- 16.Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-alpha agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain research. 2009;1268:88–96. doi: 10.1016/j.brainres.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 17.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERalpha is necessary for estradiol's anorexigenic effect in female rats. Hormones and behavior. 2010;58:872–7. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young JR, Jaffe RB. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. The Journal of clinical endocrinology and metabolism. 1976;42:432–42. doi: 10.1210/jcem-42-3-432. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125:143–9. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- 20.Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biology of reproduction. 2003;69:1771–8. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- 21.Brann DW, Mahesh VB. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Frontiers in neuroendocrinology. 1994;15:3–49. doi: 10.1006/frne.1994.1002. [DOI] [PubMed] [Google Scholar]
- 22.Ping L, Mahesh VB, Wiedmeier VT, Brann DW. Release of glutamate and aspartate from the preoptic area during the progesterone-induced LH surge: in vivo microdialysis studies. Neuroendocrinology. 1994;59:318–24. doi: 10.1159/000126673. [DOI] [PubMed] [Google Scholar]
- 23.MohanKumar SM, MohanKumar PS. Aging alters norepinephrine release in the medial preoptic area in response to steroid priming in ovariectomized rats. Brain research. 2004;1023:24–30. doi: 10.1016/j.brainres.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 24.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downs JL, Wise PM. The role of the brain in female reproductive aging. Molecular and cellular endocrinology. 2009;299:32–8. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neal-Perry G, Nejat E, Dicken C. The neuroendocrine physiology of female reproductive aging: An update. Maturitas. 2010;67:34–8. doi: 10.1016/j.maturitas.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Annals of the New York Academy of Sciences. 2000;904:502–6. doi: 10.1111/j.1749-6632.2000.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 28.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–58. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA : the journal of the American Medical Association. 2004;292:2991–6. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 30.Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biology of reproduction. 1994;51:1264–72. doi: 10.1095/biolreprod51.6.1264. [DOI] [PubMed] [Google Scholar]
- 31.Gore AC, Yeung G, Morrison JH, Oung T. Neuroendocrine aging in the female rat: the changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-methyl-D-aspartate receptors. Endocrinology. 2000;141:4757–67. doi: 10.1210/endo.141.12.7841. [DOI] [PubMed] [Google Scholar]
- 32.Gore AC, Oung T, Woller MJ. Age-related changes in hypothalamic gonadotropin-releasing hormone and N-methyl-D-aspartate receptor gene expression, and their regulation by oestrogen, in the female rat. Journal of neuroendocrinology. 2002;14:300–9. doi: 10.1046/j.1365-2826.2002.00777.x. [DOI] [PubMed] [Google Scholar]
- 33.Rivera HM, Oberbeck DR, Kwon B, Houpt TA, Eckel LA. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain research. 2009;1259:51–8. doi: 10.1016/j.brainres.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen-sensitive mechanism. Peptides. 2004;25:667–74. doi: 10.1016/j.peptides.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Murray JF, Baker BI, Levy A, Wilson CA. The influence of gonadal steroids on pre-pro melanin-concentrating hormone mRNA in female rats. Journal of neuroendocrinology. 2000;12:53–9. doi: 10.1046/j.1365-2826.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- 36.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–708. doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchino E, Tsuzuki T, Inoue K. The effects of age and sex on seven elements of Sprague-Dawley rat organs. Laboratory animals. 1990;24:253–64. doi: 10.1258/002367790780866182. [DOI] [PubMed] [Google Scholar]
- 38.Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O'Byrne KT. The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. Journal of neuroendocrinology. 2003;15:468–76. doi: 10.1046/j.1365-2826.2003.01014.x. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. Journal of neuroendocrinology. 2007;19:426–31. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 40.Diano S, Kalra SP, Sakamoto H, Horvath TL. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain research. 1998;812:256–9. doi: 10.1016/s0006-8993(98)00936-6. [DOI] [PubMed] [Google Scholar]
- 41.Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–21. doi: 10.1210/en.2004-0129. [DOI] [PubMed] [Google Scholar]
- 42.Silva LE, Castro M, Amaral FC, Antunes-Rodrigues J, Elias LL. Estradiol-induced hypophagia is associated with the differential mRNA expression of hypothalamic neuropeptides. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 2010;43:759–66. doi: 10.1590/s0100-879x2010007500059. [DOI] [PubMed] [Google Scholar]
- 43.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 44.Rivera HM, Santollo J, Nikonova LV, Eckel LA. Estradiol increases the anorexia associated with increased 5-HT(2C) receptor activation in ovariectomized rats. Physiology & behavior. 2012;105:188–94. doi: 10.1016/j.physbeh.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polidori C, Geary N. Estradiol treatment fails to affect the feeding responses to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides. 2002;23:1697–700. doi: 10.1016/s0196-9781(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 46.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–8. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 47.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–92. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 48.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behavioural brain research. 2008;191:173–7. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santollo J, Eckel LA. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiology & behavior. 2008;93:842–50. doi: 10.1016/j.physbeh.2007.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muschamp JW, Hull EM. Melanin concentrating hormone and estrogen receptor-alpha are coexstensive but not coexpressed in cells of male rat hypothalamus. Neuroscience letters. 2007;427:123–6. doi: 10.1016/j.neulet.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, et al. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology. 2010;58:314–20. doi: 10.1016/j.neuropharm.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell metabolism. 2011;14:453–65. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez-Galaz C, Fernandez-Agullo T, Campoy F, Arribas C, Gallardo N, Andres A, et al. Decreased leptin uptake in hypothalamic nuclei with ageing in Wistar rats. The Journal of endocrinology. 2001;171:23–32. doi: 10.1677/joe.0.1710023. [DOI] [PubMed] [Google Scholar]
- 54.Escobar CM, Krajewski SJ, Sandoval-Guzman T, Voytko ML, Rance NE. Neuropeptide Y gene expression is increased in the hypothalamus of older women. The Journal of clinical endocrinology and metabolism. 2004;89:2338–43. doi: 10.1210/jc.2003-031899. [DOI] [PubMed] [Google Scholar]
- 55.Sahu A, Kalra SP. Absence of increased neuropeptide Y neuronal activity before and during the luteinizing hormone (LH) surge may underlie the attenuated preovulatory LH surge in middle-aged rats. Endocrinology. 1998;139:696–702. doi: 10.1210/endo.139.2.5728. [DOI] [PubMed] [Google Scholar]
- 56.Wolden-Hanson T. Mechanisms of the anorexia of aging in the Brown Norway rat. Physiology & behavior. 2006;88:267–76. doi: 10.1016/j.physbeh.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. The Journal of comparative neurology. 2003;466:409–21. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- 58.Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, et al. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E617–24. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]