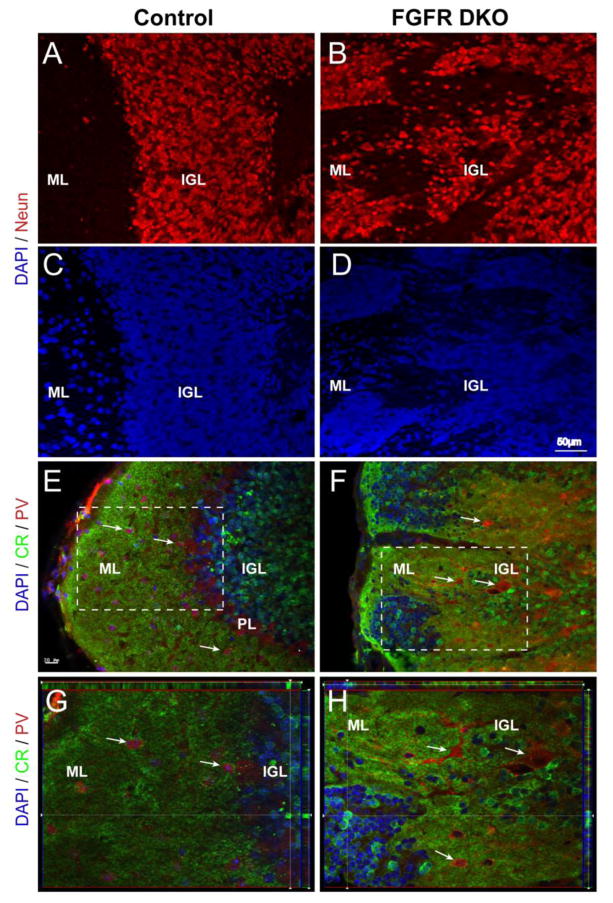
Figure 3. Clusters of misplaced cells in the cerebellar cortex of adult FGFR DKO mice are granule cell neurons.
Sagittal sections of adult control (A and C) and FGFR DKO (B and D) were immunostained for the neuronal marker NeuN (A and B), while all nuclei were stained with DAPI (C, D). The location of NeuN positive cells in the control demonstrates a clear transition from the cell dense IGL to the ML. In the mutant, labeled NeuN cells are scattered throughout the lobule with no clear demarcation between the IGL and ML. (EH) Granule cells were identified by Calretinin (CR) immunostaining and Purkinje neurons and interneurons were identified with Parvalbumin (PV) in control (E and G) and FGFR DKO mice (F and H). In the control, CR cell body staining is limited to the IGL and excluded from the ML (E, inset shown in G) whereas in the mutant cerebellum, there are Calretinin-positive cells throughout the lobule (F, inset shown in H) and in isolated pockets in the ML. The absence of a clear boundary between the IGL and ML is indicated by pockets of Calretinin+ cells located at the most external portion of the lobule. PV staining was present in the PL and in ML in control animals (E,G), in comparison to FGFR DKO mice, in which most PV+ cells were dispersed in the IGL away from the ML, with few PV cells in the molecular layer (F,H).