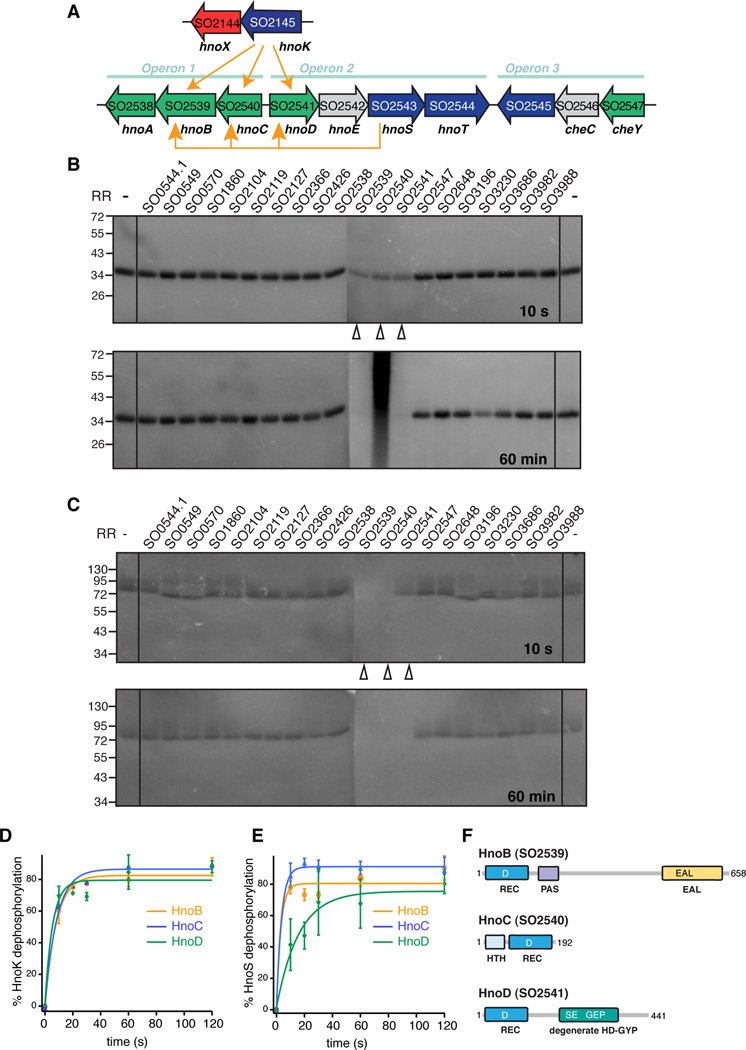
Figure 1. Identification of cognate response regulators to the H-NOX-associated histidine kinase in Shewanella oneidensis.
(A) The H-NOX gene (hnoX, SO2144, red) and the H-NOX-associated HK gene (hnoK, SO2145, purple) form an isolated operon in Shewanella oneidensis. The three cognate RR genes of the H-NOX-associated HK hnoB (SO2539), hnoC (SO2540) and hnoD (SO2541) (green) are located in three operons that are rich in two-component signaling proteins. The adjacent HK hnoS (SO2543, purple) had the same three response regulators targets (yellow arrows). (B) Phosphotransfer profiling of the H-NOX-associated HK (HnoK, SO2145) to 20 orphan RRs identified three cognate phosphorylation targets (SO2539, SO2540, SO2541). The HK was pre-phosphorylated with [γ-32P]-ATP and subsequently incubated with an equimolar amount of the respective RR for either 10 s or 60 min. Phosphorylation was detected by visualizing 32P radioactivity after SDS-PAGE (C) HnoS (SO2543) displayed the same phosphotransfer specificity for SO2539, SO2540, SO2541. (D–E) Comparison of phophotransfer kinetics of the two HKs. All values represent the mean (n = 2 or 3) +/− SEM (F) Domain architecture of cognate response regulators, displaying the receiver domain with the conserved aspartic acid (REC, dark blue) and the varying effector domains. HnoB contains a PAS domain and an EAL PDE domain (yellow). HnoC exhibits a helix-turn-helix (HTH) DNA binding domain (light blue) and HnoD contains a degenerate HD-GYP domain (green). See also Figure S1.