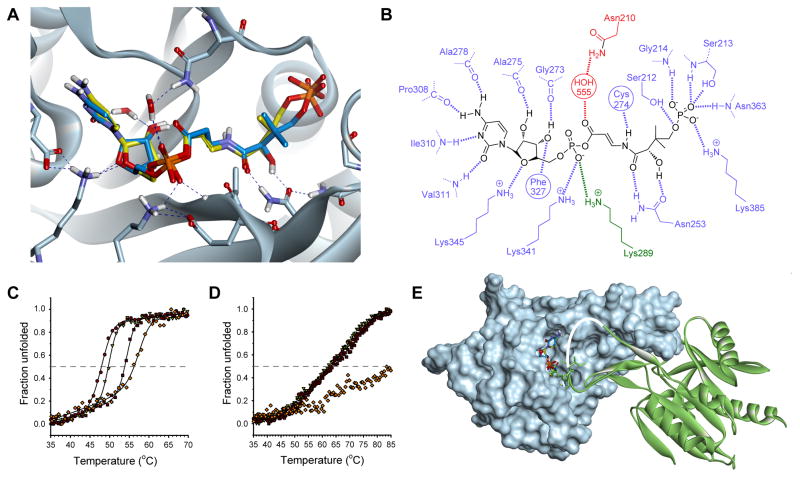
Figure 7. Structural basis for the tight-binding inhibition of the CJ-15,801-derived inhibitor.
(A) Structure of the P-CJ-CMP inhibitor 16 (stick structure with carbon atoms in yellow) modeled and overlaid on that of the P-Pan-CMP intermediate 14 (stick structure with carbon atoms in blue) bound in the active site of native EcPPCS. The model was created from the crystal structure of EcPPCS Asn210Asp with co-crystalized P-Pan-CMP 14 bound (pdb: 1U7Z) by reversing the mutation, and docking P-CJ-PMP. The highest scoring pose of P-CJ-CMP closely resembles that of P-Pan-CMP and similarly facilitates all hydrogen bonding interactions observed for P-Pan-CMP. Only selected residues are shown for clarity. See also Figure S4.
(B) Schematic view of polar interactions between P-CJ-CMP and EcPPCS. Residues from monomer A are in blue and those from monomer B in green. The key interaction between Asn210 and the acyl phosphate carbonyl, mediated via a bridging water molecule, is shown in red.
(C) Heat-induced protein melting curves for EcPPCS. The four curves represent from left to right: the free protein (●); protein with MgCTP (▽); protein with MgCTP and P-Pan (2) (■); and protein with MgCTP and P-CJ (13) (◇). The curves were determined by following changes in the protein’s secondary structure by circular dichroism.
(D) Heat-induced protein melting curves for SaCoaBC determined as for EcPPCS in (B).
(E) Structure of the EcPPCS dimer (pdb: 1U7Z) with one monomer shown as a solvent-accessible surface (in cyan) and the other as ribbon structure (in green). The overlayed P-Pan-CMP and P-CJ-CMP stick structures are shown docked in the active site. Note that residues 291 to 298, which form part of a loop (shown in white) that cover the active site, is normally disordered and is absent in all crystal structures of PPCS enzymes (Manoj, et al., 2003; Stanitzek, et al., 2004).