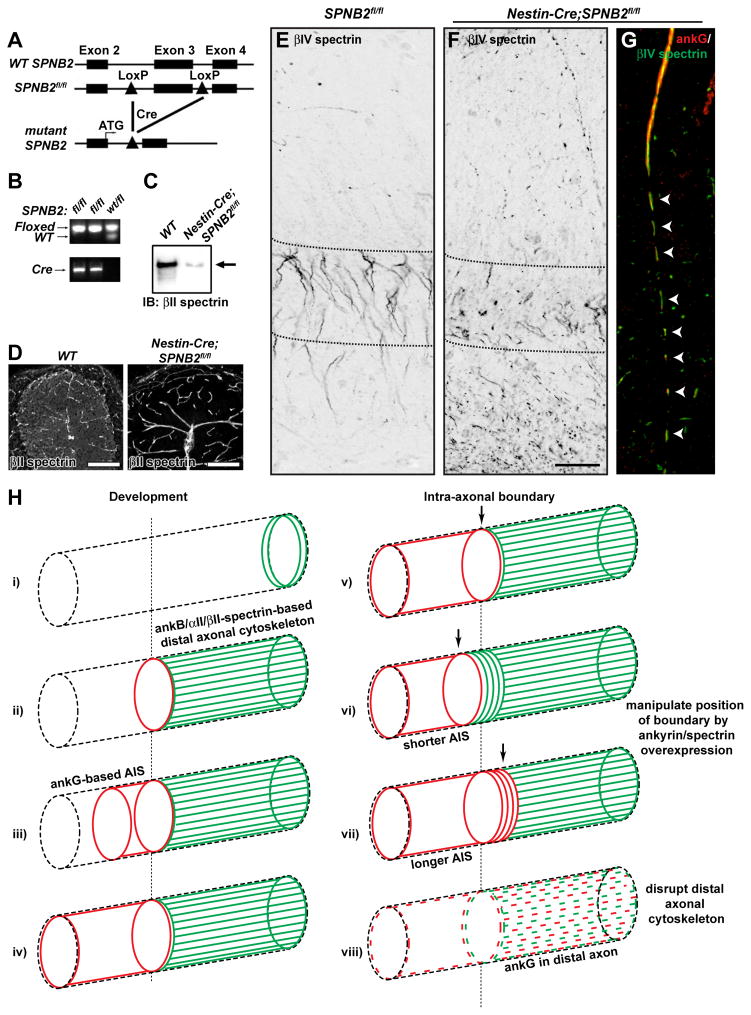
Figure 7. Analysis of a nervous system specific βII spectrin-deficient mouse.
(A) Schematic representation of the genomic wild-type SPNB2 (WT SPNB2), floxed (SPNB2fl/fl), and mutant loci.
(B) PCR analysis of genomic tail DNA of mutant (fl/fl) or heterozygous (wt/fl) mice.
(C) Immunoblot analysis of βII spectrin in brain lysates from wild-type (WT) or Nestin-Cre;SPNB2fl/fl mice.
(D) βII spectrin immunostaining of WT and Nestin-Cre;SPNB2fl/fl cerebellum. High levels of βII spectrin immunoreactivity remain in the vasculature. Scale bar, 100 μm.
(E–F) βIV spectrin labeling of SPNB2fl/fl (E) and Nestin-Cre;SPNB2fl/fl hippocampus. Scalebar, 50 μm.
(G) AnkG (red) and βIV spectrin (green) Immunostaining of Nestin-Cre;SPNB2fl/fl axon. Arrowheads indicate the fragmented AIS.
(H) i) AnkB, αII spectrin, and βII spectrin are located in the distal growth cone during axonogenesis. ii) AnkB, αII spectrin, and βII spectrin comprise a distal axonal cytoskeleton that is assembled before ankG clustering. iii) AnkG accumulates at the distal end of the future AIS, at the boundary with the distal axonal submembranous cytoskeleton. iv) AnkG fills and defines the AIS in a distal to proximal direction. v) The transition from ankG/βIV spectrin to ankB/αII spectrin/βII spectrin along the axon defines the intra-axonal boundary. vi) Overexpression of ankB causes a proximal shift of the intra-axonal boundary and a shorter AIS. vii) Overexpression of ankG causes a distal shift of the intra-axonal boundary and a longer AIS. viii) Loss of the intra-axonal boundary by disruption of the distal axonal cytoskeleton blocks AIS assembly and permits ankG to be found in the distal axon.