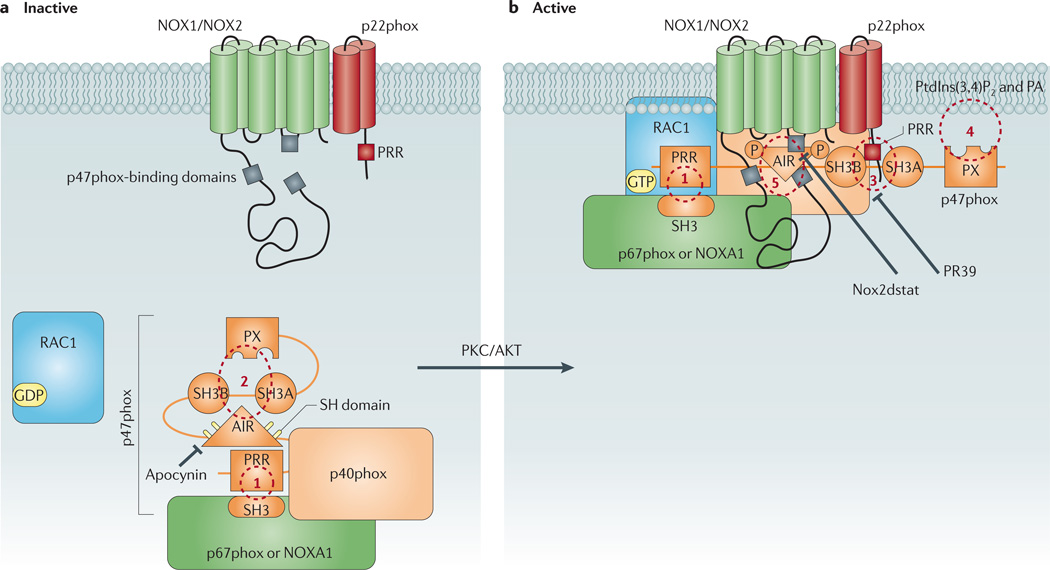
Figure 3. Schematic diagram showing p47phox as the central organizer of the vascular NOX1 and NOX2 oxidases.
a | This figure shows the conformation of p47phox in resting cells in which the protein forms a complex with other cytosolic regulatory subunits via an interaction between its carboxy-terminal proline-rich region (PRR) and an Src homology 3 (SH3) domain on one of two potential activator proteins, p67phox or NADPH oxidase activator 1 (NOXA1) subunit (site 1). In the resting state, p47phox adopts a closed conformation in which its tandem-repeat SH3 and phox homology (PX) domains are ensconced and unable to interact with the membrane components. This conformation is largely achieved via intramolecular interactions of both the polybasic autoinhibitory region (AIR) and the PX domain with the tandem-repeat SH3 domain (site 2). b | Phosphorylation of sulfhydryl groups (SH) on crucial cysteine residues within the AIR destabilizes these intramolecular interactions and causes p47phox to unfold. This exposes the tandem-repeat SH3 domain of p47phox, thereby allowing it to associate with a PRR on the amino terminus of membrane-bound p22phox (site 3), and the PX domain of p47phox, which interacts with membrane phospholipids including phosphatidylinositol-3,4-biphosphate (PtdIns(3,4)P2) and phosphatidic acid (PA) (site 4). The association of the cytosolic complex with the membrane subunits is further stabilized by direct protein–protein interactions between p47phox and the NOX2 subunit (site 5). It is unclear whether p47phox undergoes similar protein–protein interactions with the NOX1 subunit. Also shown are likely target sites for conventional NADPH oxidase inhibitors. PKC, protein kinase C.